On May 5, 2025, Yaou Shen's team from Sichuan Agricultural University published a research paper titled "Dynamic transcriptome and GWAS uncover that a hydroxyproline-rich glycoprotein suppresses Agrobacterium-mediated transformation in maize" in Molecular Plant. The study identified key genes involved in Agrobacterium-mediated transformation of maize immature embryos and established an efficient maize genetic transformation system.
Maize (Zea mays L.), as the world's highest-yielding food crop, occupies a core position in food processing, animal husbandry and bioenergy. Improving maize yield, quality and environmental adaptability through modern biobreeding technology is an important research direction for maize genetic improvement.
Transgenic and gene editing are revolutionary technologies in the field of maize biological breeding, which are highly dependent on Agrobacterium-mediated genetic transformation of immature embryos. However, most maize germplasms have low genetic transformation rates and cannot be directly used as transformation receptors, becoming a bottleneck factor in maize biological breeding.
Mining the key genes regulating Agrobacterium transformation in maize is of great significance for establishing efficient genetic transformation and genome editing systems for excellent maize lines and accelerating the process of maize genetic improvement.
In order to eliminate the genotype dependence of maize genetic transformation, this study independently developed a precise quantitative method for Agrobacterium infection rate, and integrated dynamic transcriptome and genome-wide association analysis (GWAS) to identify the variant sites and key genes regulating Agrobacterium infection rate (AIF) in maize. Through dynamic transcriptome analysis of maize immature embryos during Agrobacterium infection and co-cultivation, 8,483 and 1,580 genotype-specific response genes were identified in the low infection rate inbred line 18-599R and the high infection rate inbred line A188, respectively.
Weighted gene co-expression network analysis (WGCNA) further revealed that there were 5 and 7 Agrobacterium co-cultivation stage-specific expression modules in the two inbred lines, respectively. At the same time, GWAS was performed by combining the genotypes and two-environment phenotype data of 310 inbred lines, and 30 single nucleotide polymorphisms (SNPs) and 315 candidate genes were associated. The integration of GWAS and WGCNA results screened out 12 key genes closely related to the high infection rate of A188.
CRISPR/Cas9-mediated gene knockout proved that the hydroxyproline-rich glycoprotein ZmHRGP is a key factor in negatively regulating the infection rate of maize immature embryos. After knocking out ZmHRGP in 18-599R, the Agrobacterium infection rate of immature embryos increased from 36% to 90%, and the transformation rate increased from 11% to 80%. In addition, transient inhibition of ZmHRGP expression by RNAi can also significantly increase the Agrobacterium infection rate of maize callus and leaves.
This study improves the molecular mechanism of plant response to Agrobacterium infection and provides a new strategy for establishing a genotype-universal maize genetic transformation system.
Phenotypic evaluation of Agrobacterium infection rate of immature embryos is difficult. In order to accurately evaluate the infection rate of maize immature embryos, this study independently developed a quantitative method based on enhanced green fluorescent protein (eGFP) expression. The first intron of the maize ubiquitin gene ZmUbiquitin1 was inserted into the eGFP coding region to transform it into eGFP-intron, which was introduced into Agrobacterium and infect maize immature embryos. eGFP-intron does not express eGFP protein in Agrobacterium cells that lack intron splicing mechanism. It can only be expressed normally when the eGFP gene is introduced into immature embryo cells. Therefore, the green fluorescence detected under a fluorescence microscope is the eGFP protein expressed in the immature embryo.
This design effectively avoids the contamination of maize immature embryo cell fluorescence by Agrobacterium cell fluorescence. At the same time, the intron-spanning eGFP quantitative primers were designed to perform RT-qPCR analysis of eGFP on the infected young embryos to avoid the influence of the eGFP-intron sequence carried by Agrobacterium on the expression of mature eGFP mRNA in young embryo cells, and to achieve quantitative Agrobacterium infection rate by RT-qPCR of eGFP in young embryos. Finally, the fluorescence density of young embryos was combined with the abundance of mature eGFP mRNA to accurately evaluate the infection rate of maize young embryos.
Using young embryos of inbred lines 18-599R (low AIF) and A188 (high AIF) with significant differences in infection rate phenotypes, dynamic sampling was performed during Agrobacterium infection and co-cultivation for comparative transcriptome analysis. Through WGCNA and genotype-specific co-expression module analysis, 1261 genes that specifically responded to Agrobacterium infection in A188 were identified.
Using the AIF quantitative method established in this study, the researchers evaluated the phenotypes of 310 maize inbred lines in two environments, Xishuangbanna and Chengdu, including AIF at 36 h, 48 h, and 72 h after infection. GWAS identified 30 AIF-related SNPs and annotated 315 candidate genes. Combined with the A188-specific response genes identified by the above WGCNA, a total of 12 candidate genes related to infection rate were obtained. Among them, the gene ZmHRGP, which was consistently low expressed in the high-AIF inbred lines A188, C01, and B104, and highly expressed in the low-AIF inbred line 18-599R, received further attention.
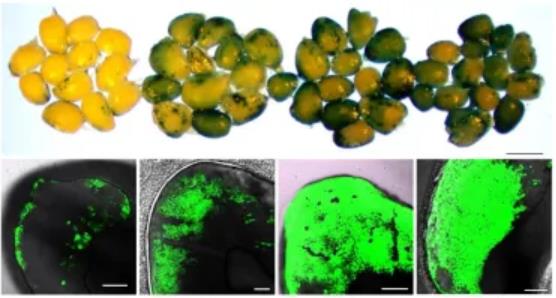
Figure 1. After knocking out ZmHRGP, the Agrobacterium infection rate of maize embryos was significantly increased. (Liu, et al., 2025)
The CRISPR/Cas9 system was used to knock out the ZmHRGP gene in the low-AIF inbred line 18-599R, which greatly improved the Agrobacterium infection efficiency. In addition, based on the ZmHRGP knockout material, this study established an efficient Agrobacterium-mediated genetic transformation system, and the transformation rate of young embryos increased from 11% to 80%. At the same time, this study also used RNAi to inhibit the expression of ZmHRGP, verifying that transient inhibition of ZmHRGP expression can also significantly improve the Agrobacterium infection efficiency of corn callus and leaves.