Plant physiology is a discipline of studying the variety of biological processes and functional activities occurring in plants. Plant physiological processes related to photosynthesis and water use provide organic compounds and energy for their own growth, as well as the food source for other organisms. Improving photosynthetic capacity and efficiency are essential for increasing the yield potential of crops.
Lifeasible, as a leading plant biotechnology company, provides services for the measurement of traits regarding plant photosynthesis and water use, with both high-throughput and conventional methods. We are devoted to offering our customers worldwide with highly accurate and repeatable data.
Photosynthesis and respiration rate
Photosynthesis is a process in which green plants use energy from the sun to transform water, carbon dioxide, and minerals into oxygen and sugar (Figure 1). The evaluation of photosynthesis rate can be based on uptake of carbon dioxide, production of oxygen, production of carbohydrates, or increase of dry biomass. Opposite to the photosynthesis process, the respiration process utilizes the sugar and oxygen to produce energy for plant growth (Figure 1). Therefore, respiration rates are usually reflected by volume of carbon dioxide produced, volume of oxygen consumed, or fresh product consumed within a certain period of time.
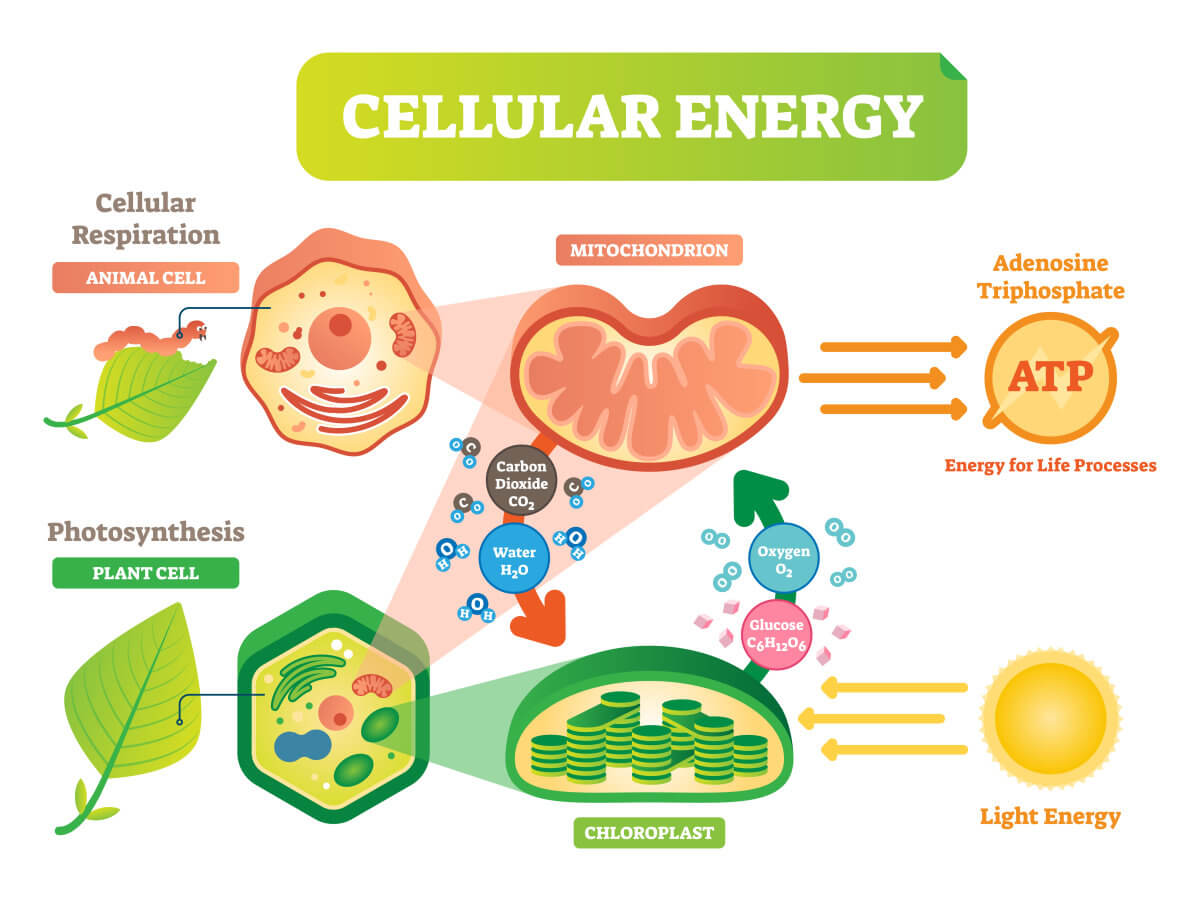 Figure1. The processes of photosynthesis and respiration.
Figure1. The processes of photosynthesis and respiration.
Parameters reflecting the photosynthesis and respiration rate can be measured by multiple portable photosynthesis systems with non-dispersive infrared gas analyzers (IRGA), such as Li-6400, Li-6400XT, Li-6800, CI-340, LCi-SD, and so on. In addition, we offer carbon isotope analysis for photosynthesis pathway determination. This method relies on the fact that photosynthetic enzymes (ribulose bisphosphate carboxylase) discriminate against the heavier stable isotope 13C (relative to 12C) during photosynthesis, so that C in leaves is always depleted in 13C compared with that in the atmosphere. The isotope ratio mass spectrometer (δ13C) for C3, C4, facultative crassulacean acid metabolism (CAM), and obligate CAM photosynthesis is -21‰ to-35‰,-10‰ to -14‰, -15‰ to -20‰, and -10‰ to-15‰, respectively.
Leaf nitrogen content
Nitrogen plays a key role in the plant life cycle. It is the main mineral nutrient resource for the production of chlorophyll and other plant cell components (proteins, nucleic acids, amino acids). Therefore, the measurement of leaf nitrogen content is important for the evaluation of photosynthesis rate and crop yield.
We offer multiple methods for monitoring leaf nitrogen status (Figure 2), including:
 Figure 2. Methods for plant nitrogen sensing (Muñoz-Huerta et al., 2013).
Figure 2. Methods for plant nitrogen sensing (Muñoz-Huerta et al., 2013).
Stomatal conductance
Stomatal conductance reflects the rate of gas exchange (i.e., uptake of carbon dioxide) and water loss (i.e., transpiration) through the leaf stomata. It can indicate the degree of stomatal opening and plant water status. Stomatal conductance is usually expressed in mmol m-2 s-1, and can be measured using different types of leaf porometers:
Transpiration rate
Transpiration is a process that leads to water loss through the stomata of plants via evaporation. Transpiration not only promotes uptake of water and mineral nutrients from the soil, but also help cools down the plants. The transpiration rate for a leaf with more opened stomata is potentially higher.
At Lifeasible, a number of techniques, including photometers (Figure 3), lysimeters, porometers (e.g., SC-1 or AP4), photosynthesis systems (e.g., Li-6400, Li-6400XT, Li-6800, CI-340, LCi-SD) and thermometric sap flow sensors, are available for the measurement of transpiration rate. The transpiration rate can also be measured by carbon isotope discrimination. Due to the different diffusivities of 13C and 12C across the stomata, and the fractionation by the photosynthetic enzyme, the ratio of 13C to 12C can be discriminated by the mass spectrometer, and the transpiration rate can be calculated accordingly.
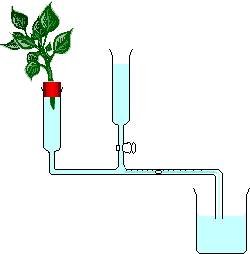 Figure 3. A diagram of bubble photometer for transpiration rate measurement (from Wikipedia).
Figure 3. A diagram of bubble photometer for transpiration rate measurement (from Wikipedia).
Water Use Efficiency
Water use efficiency (WUS) reflects carbon gain per unit water loss. The instantaneous WUS can be calculated as the ratio of photosynthesis rates to transpiration rates or stomatal conductance. On the other hand, long-term WUS is evaluated by transpiration rate via the carbon isotope approach. In general, higher values indicate higher WUS.
Leaf water potential
Leaf water potential is a simple indicator of leaf water levels. The more negative the value, the more dehydrated the leaf is. We provide three methods for leaf water potential measurement.
Reference