Analysis of Plants Endoplasmic Reticulum Stress Response
Endoplasmic reticulum (ER) is an important site for synthesizing new peptide chains by a membrane and secreted proteins, and also contains the initial folding modification of new peptide chains. But folding and modification process is complex and error-prone; misfolded proteins in the endoplasmic reticulum quality control (ERQC) system to identify and trap within the endoplasmic reticulum. Once the accumulation of erroneous proteins exceeds the capacity of ER, a series of cellular responses will be triggered to achieve a new ER homeostasis, which is called ER stress response.
Lifeasible, as a leading global company, is committed to helping our customers achieve effective and successful research. We provide analysis of endoplasmic reticulum stress response in plants, including endoplasmic reticulum quality control, unfolded protein response, endoplasmic reticulum quality control, autophagy of endoplasmic reticulum quality control, and others. We always deliver reliable results and reports on time to our customers worldwide.
- Protein folding in the ER is high-speed, complex, and error-prone. In order to ensure that only correctly folded proteins enter the Golgi, there is a special system in the ER responsible for monitoring the level of protein folding called the ER quality control system.
- Lifeasible helps our client analyze the key genes in ER quality control systems, such as CNX / CRT, BRI, UGGT, MNS, etc. We provide quality services in the research process, including the development of double mutants, multiple mutants, transient expression of tobacco, homologous gene analysis, plant phenotype analysis, and so on.
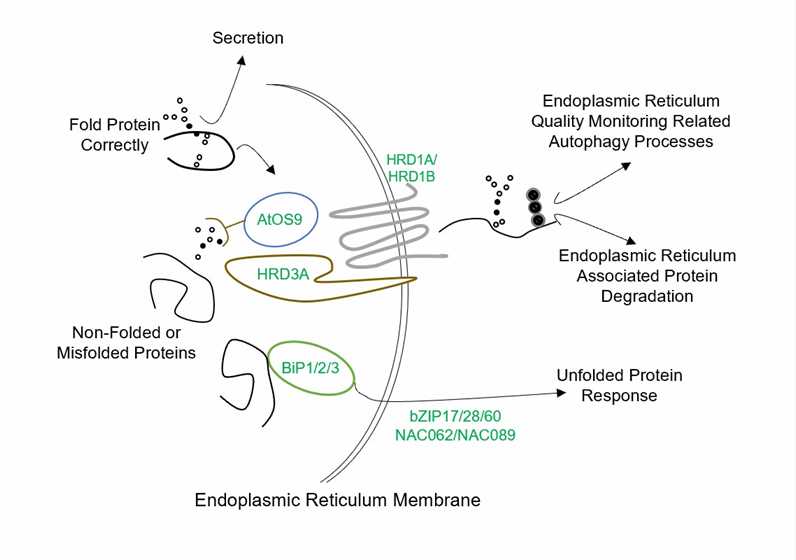 Fig.1 Endoplasmic reticulum quality control system in plants.
Fig.1 Endoplasmic reticulum quality control system in plants.
- Large accumulation of misfolded proteins in the ER induces unfolded protein response (UPR). The UPR process is also conserved in yeast, animals, and plants, but the specific mechanism is different.
- We help our customers to analyze UPR pathways in plants; for example, one pathway is mediated by bZIP60 transcription factor, and the other pathway is mediated by bZIP28 transcription factor.
- To alleviate ER stress, the direct removal of misfolded proteins is also crucial for organisms. The ERAD mechanism reverses the misfolded protein substrate to the cytoplasm for ubiquitination and finally clears it by the cytoplasmic 26S proteasome.
- We help study this process, for example, OS9 recognizes misfolded glycoproteins, E3 ligases such as HRD, DOA10 and Rmal, adaptor protein HRD3A, and ubiquitin binding enzyme UBC32 modify misfolded proteins by ubiquitination and then degradation.
- Larger membrane protein molecules are degraded through the autophagy pathway.
- Although there are few researches on this part, and IRE1b may play an important role in this process, we help customers explore more mysteries with our professional team and advanced experimental platform.
Lifeasible is always devoted to providing high-quality and satisfactory service to our customers. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
For research or industrial raw materials, not for personal medical use!
 Fig.1 Endoplasmic reticulum quality control system in plants.
Fig.1 Endoplasmic reticulum quality control system in plants.