Fungi can secrete a large number of enzymes to break down the substances in their environment. The mixture of enzymes secreted by fungi is of great use in many industrial processes. Maximizing the production of secreted enzymes is of great importance. Traditional mutagenesis and screening methods have produced the majority of strains used for the industrial production of enzymes. With the increasing application of DNA recombination technology, protoplast fusion, and histological studies, new methods and strategies for strain improvement are emerging. For those strains with clear biochemical pathways, strain improvement targets can be selected by metabolic engineering strategies, and for strains with unclear biochemical metabolic pathways, genetic selection can also be performed directly by genomic recombination, systems biotechnology, ribosome engineering, and epigenetic modification to obtain desirable mutant phenotypes. Lifeasible is dedicated to making fungal selective breeding more directed and ortho-mutagenic, offering a variety of new methods for constructing highly enzyme-producing fungal strains.
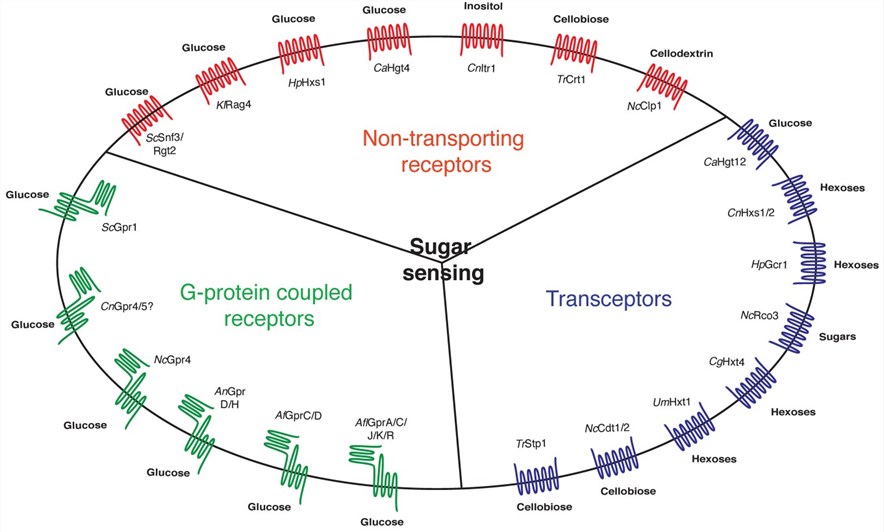 Figure 1. Sugar-sensing proteins in the plasma membrane of fungal cells. (Van Dijck P, et al., 2017)
Figure 1. Sugar-sensing proteins in the plasma membrane of fungal cells. (Van Dijck P, et al., 2017)
We use various genetic manipulation techniques to control the expression of multiple genes. These genes are involved in processes that affect the ability of the fungus to sense its environment and regulate the transcription of enzyme-encoding genes and the secretion of proteins. We are committed to using these techniques to rationally engineer improved fungi to provide high fungal nutrient sensing, gene transcription, protein translation, and secretion capabilities.
Lifeasible is committed to using genetic modification technologies to help customers build high enzyme-producing fungal strains by improving the nutrient sensing, gene translation, protein transcription, and secretion capabilities of fungi. As your trusted partner, we can meet all your fungal phylogenetic analysis needs and provide efficient, high-quality services. If you want to know the details, please contact us.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
STU-CRISPR System Improves Plant Genome Editing Efficiency
April 19, 2024
Application of Exosomes in Facial Beauty
April 12, 2024