Biomolecular Fluorescence Complementation (BiFC) is a powerful tool for the validation of protein-protein interactions. The greatest advantage of BiFC is that, unlike other complementation methods, it allows direct visualization of protein interactions, as well as their subcellular localizations, in living cells (1). As a molecular biology based assay that is straightforward to perform both in cell cultures and in tissues, BiFC has been applied in the studies of proteins from a variety of cell types and organisms from different species, including plants (2, 3). Here at
Lifeasible, we provide top-notch BiFC services to facilitate your research in plant cell lines, plant tissue culture, as well as in plant organs, including leaves, roots, and elongating hypocotyl cells.
The principal of BiFC is based on the reconstitution and activation of a fluorophore reporter. Specifically, two non-fluorescent fragments (FP-N and FP-C) of a fluorescent protein (FP) are fused to the proteins to be tested (protein A and B), respectively. Upon physical interaction between protein A and B, the two non-fluorescent fragments are brought into close proximity, which enables reconstitution of a functional FP complex (Figure 1). Subsequently, the fluorescence signal can be detected via fluorescence microscope or flow cytometer.
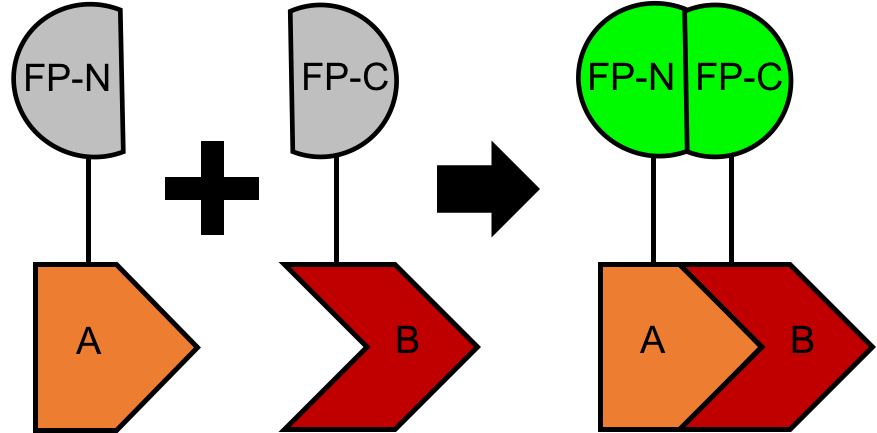 Figure 1. A schematic cartoon of the BiFC assay.
Figure 1. A schematic cartoon of the BiFC assay.
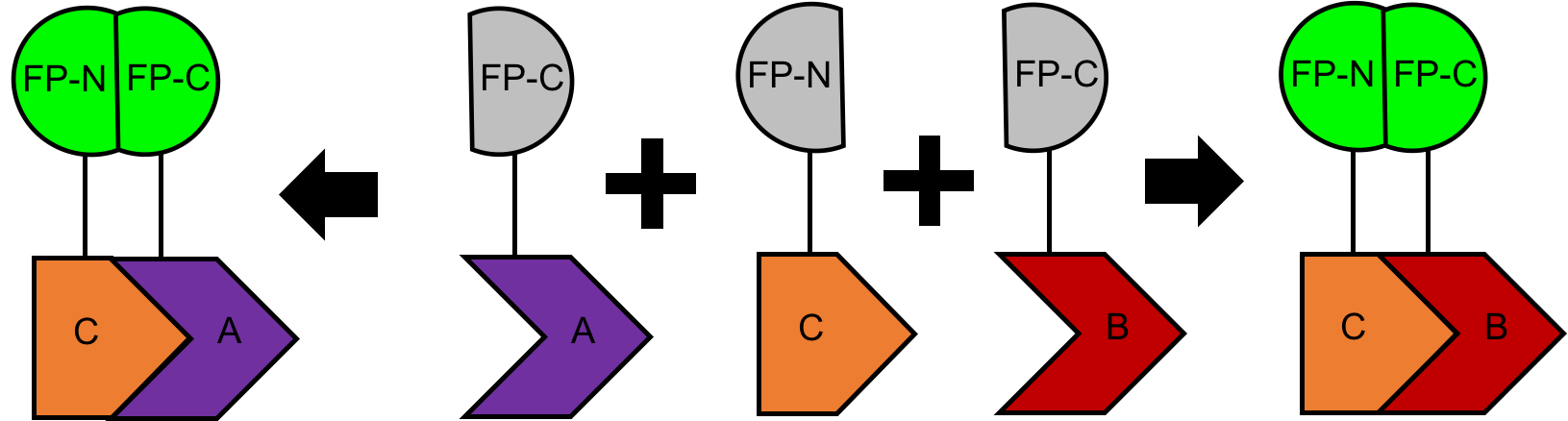 Figure 2. BiFC assay designed for detecting multiple protein interactions
Figure 2. BiFC assay designed for detecting multiple protein interactions
Compared to other assays for the investigation of protein-protein interactions, the BiFC assay has the following intrinsic advantages (4):
- Allows direct visualization of protein interactions in living cells
- Allows parallel examination of multiple protein interaction pairs (Figure 2)
- Provides strong fluorescence signal that is easy to detect
- Applicable for a wide range of proteins with different characteristics
- Fairly low threshold for protein expression
- Allows detection of interactions between protein fragments
After years of collaborative work aiming for optimized BiFC assays—with a broader range of spectrum and reduced background,
Lifeasible now provide BiFC services for numerous plant systems with enhanced reliability and cost efficacy. Our experts offer technical support that covers every step of the BiFC assay, from fluorescent tagging to result reporting and consulting. Our customer centered service ensures that the needs of each project can be met.
References
- Kerppola TK (2008) Bimolecular fluorescence complementation (BiFC) analysis as a probe of protein interactions in living cells. Annu Rev Biophys 37:465-487.
- Ohad N & Yalovsky S (2010) Utilizing bimolecular fluorescence complementation (BiFC) to assay protein-protein interaction in plants. Methods Mol Biol 655:347-358.
- Schutze K, Harter K, & Chaban C (2009) Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods Mol Biol 479:189-202.
- Miller KE, Kim Y, Huh WK, & Park HO (2015) Bimolecular Fluorescence Complementation (BiFC) Analysis: Advances and Recent Applications for Genome-Wide Interaction Studies. J Mol Biol 427(11):2039-2055.
For research or industrial raw materials, not for personal medical use!
 Figure 1. A schematic cartoon of the BiFC assay.
Figure 1. A schematic cartoon of the BiFC assay. Figure 2. BiFC assay designed for detecting multiple protein interactions
Figure 2. BiFC assay designed for detecting multiple protein interactions