Reactive oxygen species are key signals for plant adaptation to environmental stresses such as drought. Roots respond to transient water shortages through the xerobranching, a temporary cessation of branching. However, the molecular mechanisms underlying this response remain largely unexplored.
On June 12, 2025, Ari Sadanandom's team from Durham University published a research paper titled "Redox-regulated Aux/IAA multimerization modulates auxin responses" in Science. This study reveals for the first time the molecular mechanism by which plant roots dynamically regulate xerobranching by triggering the multimerization of the auxin-repressing protein IAA3 through reactive oxygen species (ROS). When roots sense localized water shortage, a nuclear ROS burst mediated by RBOH oxidase prompts IAA3 to form multimers around specific cysteine residues, enhancing its ability to recruit the co-repressor TOPLESS, thereby inhibiting lateral root development. This discovery clarifies the critical role of ROS as an early "alarm signal" linking environmental water changes with the auxin signaling pathway, providing a new perspective for understanding plant phenotypic plasticity.
Plant roots adapt to soil moisture heterogeneity through the xerobranching (temporarily inhibiting lateral root formation). This process was previously thought to be primarily mediated by abscisic acid (ABA). However, ABA signaling cannot explain the rapid sensing of water fluctuations in roots (e.g., suspension of branching within hours). Research suggests that ROS may serve as a precursor signal under drought stress, but how it interacts with core hormone pathways such as auxin remains unknown. Auxin-repressed proteins Aux/IAAs (such as IAA3) regulate lateral root development by repressing target gene expression. Whether ROS regulates the function of these proteins through redox modifications is a key scientific question in deciphering the rapid drought branching response.
This study first demonstrated, through genetic and cell biological experiments, that xerobranching depends on ROS produced by RBOH oxidase. Using rboh mutants and ROS inhibitors, they demonstrated that ROS deficiency leads to xerobranching defects. Furthermore, using subcellular localization sensors, they captured for the first time a ROS burst within the kernel of roots within one hour of water deprivation, predating ABA accumulation.
Furthermore, tissue mapping and gene knockout experiments revealed IAA3 as a key target of ROS. Sections of the study demonstrated ROS accumulation in the endodermis/stele (lateral root initiation zone). CRISPR-generated iaa3 mutants and loss-of-function alleles abolished xerobranching ability, while iaa28 exhibited no such phenotype, confirming IAA3's specific regulatory role.
Finally, biochemical and structural biology approaches elucidated the molecular mechanism of ROS-IAA3 multimerization. Cysteine mutants and mass spectrometry analysis revealed that Cys112/169/187 mediate redox-dependent multimerization, which is abolished in the RBOH mutant background. Furthermore, exogenous H2O2 only promotes multimerization of wild-type IAA3. Furthermore, 4C/S-IAA3 in transgenic plants lost its xerobranching function and was unable to bind the co-repressor TOPLESS, leading to dysregulated expression of the downstream gene LBD16. This ultimately elucidates a novel signaling pathway: ROS-triggered IAA3 multimerization, enhanced TPL recruitment, and repressed auxin-responsive genes.
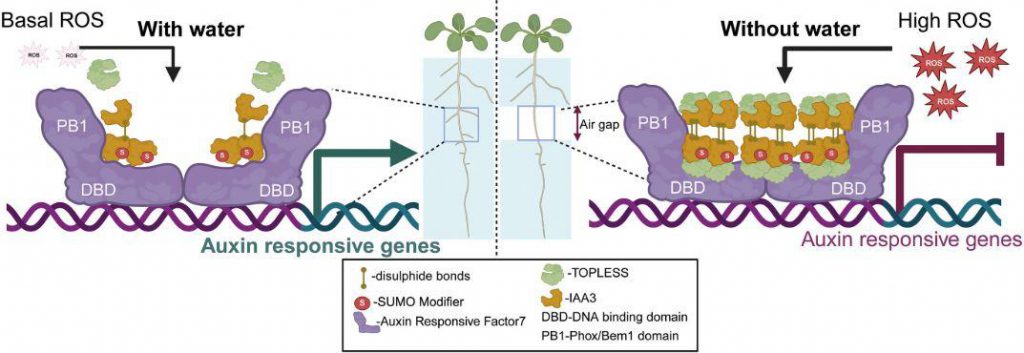
Figure 1. Model of the proposed mechanism for ROS-modulated IAA3 multimerization in mediating the repression of lateral root development during xerobranching. (Roy, et al. 2025)
In summary, this study mechanistically elucidates a rapid drought response pathway that precedes ABA, filling a critical gap in environmental signal transduction. It proposes a new paradigm for "redox-dependent transcription factor multimerization," providing a universal model for understanding ROS-regulated gene expression. Furthermore, targeting the IAA3 multimerization interface can precisely regulate root architecture, providing a new target for developing drought-resistant and water-saving crops.