A Comprehensive Guide to Plant Transformation
Basic Knowledge and Concepts of Plant Transformation
Plant genetic transformation refers to the transfer, integration, expression, and stable inheritance of a target gene into a plant through physical, chemical, or biological methods, resulting in the recipient plant expressing the traits regulated by the target gene. The core of plant transformation technology lies in the efficient transfer and stable expression of exogenous genes. Its development integrates the multidisciplinary achievements of molecular biology, cell biology, and biotechnology. From the early Agrobacterium-mediated method to modern gene editing technology, plant transformation has gradually evolved from a basic research tool to a key tool in agriculture and biotechnology.
Plant transformation can be categorized as stable transformation or transient transformation. Stable transformation occurs when a foreign gene integrates into the plant genome and is stably inherited by progeny. It is typically achieved through Agrobacterium-mediated transformation (such as T-DNA integration of a Ti plasmid). Transient transformation involves only temporary expression of the foreign gene without genomic integration and is often used for rapid functional verification (such as promoter activity analysis). For example, the floral dip method uses Agrobacterium to infect Arabidopsis floral organs, achieving stable transformation. Transient transformation, on the other hand, involves direct DNA delivery into the cell nucleus using a gene gun or electroporation.
The core mechanism of plant transformation relies on the Ti plasmid system of Agrobacterium tumefaciens. The virulence region of the Ti plasmid is activated by plant signals, translocating the Transfer DNA (T-DNA) into the plant cell nucleus and integrating it into the genome. The T-DNA typically contains the target gene, a selectable marker (such as a resistance gene), and border sequences to ensure stable expression. Furthermore, transient transformation relies on the transient expression of single-stranded T-DNA, eliminating the need for genomic integration.
Plant transformation technology began with research on Agrobacterium in the 1980s. Early methods, such as Ti plasmid modification, gradually evolved into efficient and stable transformation systems, while direct DNA transfer techniques such as gene guns and electroporation also matured. In recent years, gene editing technologies such as CRISPR-Cas9 have been integrated into transformation processes, further improving precision and efficiency.
Cross-species Gene Transfer
Cross-species gene transfer involves the introduction of foreign genes into non-traditional recipient plants, overcoming species limitations. Plant transformation techniques have expanded to encompass a wide range of species. For example, Agrobacterium-mediated transformation has been successfully applied to dicotyledonous and monocotyledonous plants such as rice, maize, and tobacco, while gene guns and electroporation are suitable for monocotyledonous crops (such as wheat and maize) that are difficult to transform with Agrobacterium. Furthermore, transformation cases in model plants (such as Arabidopsis thaliana) and commercial crops (such as potato and sweet potato) demonstrate that these techniques can be adapted to the physiological characteristics of different species.
- Petunia Model: Foreign genes have been successfully introduced into petunia through Agrobacterium-mediated transformation, making it an ideal platform for studying plant metabolic engineering and vaccine production.
- Cereal Crop Transformation: Although transformation of monocotyledonous plants is relatively challenging, efficient transformation of crops such as rice and wheat has been achieved through optimized Agrobacterium infection conditions or the combination of gene editing techniques.
- Viral-mediated Transformation: Transient expression using viral vectors (such as Cauliflower Mosaic Virus (CMV)) allows for rapid verification of gene function, but caution is required regarding the effects of viruses on plant growth.
Plant Transformation Methods and Experimental Procedures
Plant Transformation Methods
Agrobacterium-Mediated Transformation
Basic Principles and Applications
Agrobacterium-mediated transformation is one of the most commonly used techniques in plant genetic engineering. It integrates T-DNA (tumor-inducing plasmid) fragments into the plant genome via A. tumefaciens. This method relies on the natural infection mechanism of Agrobacterium: when plant tissue is wounded, Agrobacterium secretes toxic proteins that activate plant cell defense responses, leading to T-DNA entry and integration into the genome.
Experimental Procedures and Optimization
- Material Preparation: Use sterile plant tissue (such as leaves, embryos, or callus) as explants. For example, in A. thaliana, the "floral dip" method allows Agrobacterium suspensions to be applied directly to unopened inflorescences, achieving efficient transformation using egg cells as targets.
- Co-cultivation and Screening: After co-cultivation with Agrobacterium, the explants are transferred to a selection medium containing a selectable marker gene (such as a herbicide or antibiotic resistance) to screen for cells that have successfully integrated the foreign gene.
- Regeneration and Verification: Tissue culture techniques are used to induce callus tissue to differentiate into shoots and roots, ultimately regenerating complete plants. For example, in wheat transformation, embryos or callus tissue can be used as explants, achieving transformation efficiencies exceeding 35%.
Improvement Strategies
- Simplified Process: The "floral dip" method for A. thaliana eliminates the need for tissue culture and simply involves immersing the plant in a solution containing Agrobacterium, significantly reducing operational complexity.
- Tissue Selection: Transformation efficiency is higher in young and tender tissues (such as immature embryos and callus).
- High-Throughput Transformation: Transformation efficiency can be improved by optimizing Agrobacterium concentration, adding surfactants (such as Silwet L-77), and adjusting the pH. For example, sucrose and surfactants are key factors in Arabidopsis transformation.
Biolistic (Gene Gun) Transformation
Principle and Application
The biolistic method uses high pressure to accelerate DNA-encapsulated gold or tungsten microparticles into plant tissue, directly penetrating the cell wall and releasing the DNA. This method is suitable for plants that are difficult to transform with Agrobacterium (such as monocots).
Experimental Procedure
- DNA Vector Preparation: The target DNA is bound to the metal microparticles to form a DNA-microparticle complex.
- Bombardment and Screening: Plant tissue is placed in a bombardment chamber, and microparticles are injected into cells using a high-voltage pulse. Transformed cells are then screened on a resistant culture medium.
- Regeneration and Verification: Tissue culture techniques are used to induce callus tissue to differentiate into complete plants.
Advantages and Limitations
- Advantages: Suitable for difficult-to-transform plants (such as monocots), without the need for host cell co-culture.
- Limitations: Potential physical damage requires optimization of pressure parameters to minimize cell death.
Virus-mediated Transformation
Principle and Mechanism
The replication mechanism of plant viral vectors (such as CMV) is utilized to insert the target gene into the viral genome and achieve transient expression through plant infection.
Experimental Procedure
- Virus Construction: The target gene is inserted into the multiple cloning site of the viral genome, replacing the reporter gene (such as GFP).
- Plant Inoculation: The virus enters plant cells through mechanical inoculation or Agrobacterium-assisted infection.
- Transient Expression: After viral replication, the target gene is expressed in the cell nucleus without integrating into the genome.
Application
Commonly used for functional gene studies (such as promoter activity analysis and protein localization).
Polyethylene Glycol (PEG)-mediated Transformation
Principle and Mechanism
PEG disrupts the cell membrane in the presence of divalent cations, allowing DNA to enter the cell directly, making it suitable for protoplast transformation.
Experimental Procedure
- Protoplast Preparation: Protoplasts are obtained by enzymatically removing the cell wall (e.g., using HNO3).
- PEG Treatment: DNA is mixed with a PEG solution, incubated, and transformed cells are isolated by centrifugation.
- Regeneration Culture: Inducing cell division and differentiation on regeneration medium.
Limitations
Low efficiency, requiring strict control of PEG concentration (usually 0.5-1%).
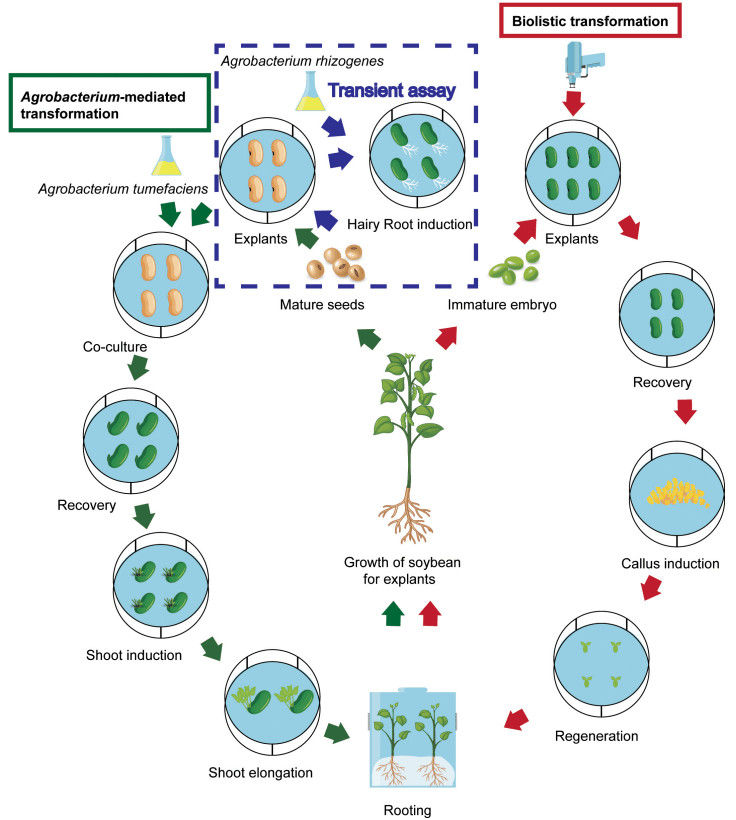 Fig. 1. General procedure of Agrobacterium-mediated and biolistic soybean transformation. (Xu, et al. 2022)
Fig. 1. General procedure of Agrobacterium-mediated and biolistic soybean transformation. (Xu, et al. 2022)
Plant Transformation Laboratory Practice Guide
- Tissue Culture: Strict control of culture medium components (e.g., hormone ratio, pH) and sterile conditions are required to promote callus differentiation and regeneration.
- Protoplast Transformation: Protoplasts are isolated by enzymatic hydrolysis, DNA is introduced via electroporation or PEG-mediated methods, and transformed cells are subsequently screened.
- Troubleshooting: Low transformation efficiency may be caused by insufficient Agrobacterium concentration, loss of plant resistance genes, or inappropriate culture conditions. Parameters may need to be adjusted (e.g., extending co-culture time, optimizing antibiotic concentration).
Plant Transformation Experimental Design and Procedures
- Purpose-driven: Select the method based on the experimental goal, e.g., stable transformation for resistance breeding, transient transformation for functional studies. Differences between monocots and dicots also lead to different strategies. Dicots (e.g., Arabidopsis) have high transformation rates, while monocots (e.g., maize) require more complex strategies.
- Explant Selection: Choose the appropriate explant based on the plant species and target gene. For example, leaves or inflorescences are commonly used in Arabidopsis, while embryos or callus tissue is often used in wheat.
- Optimization Conditions: Improve transformation efficiency by adjusting parameters such as Agrobacterium concentration, co-cultivation time, and culture medium composition.
- Molecular Verification: Confirm T-DNA integration and expression through methods such as PCR, Southern blot, and GUS/GFP reporter gene analysis.
Monocot vs. Dicot Transformation Strategies
- Dicots: Agrobacterium-mediated transformation is dominant and offers high regeneration efficiency. For example, Arabidopsis and tobacco can be transformed efficiently using Agrobacterium or floral dip methods.
- Monocots: Transformation relies on gene guns, electroporation, or PEG-mediated methods, often requiring multiple selections to obtain transformed cells. For example, wheat and rice require methods such as biolistic or protoplast transformation due to their poor tissue regeneration capacity.
Plant Tissue Culture and Regeneration
Callus Culture
Callus tissue is induced from explants, which then differentiates into shoots and roots. For example, after tobacco leaves are transformed, callus can be regenerated into complete plants.
Key Steps
- Callus Induction: Cell division is induced using hormones (such as 2,4-D and BA).
- Organogenesis: Adjusting hormone ratios (e.g., high cytokinin promotes shoot differentiation, high auxin promotes root formation).
- Plant Regeneration: Screening for resistant calli and transferring them to differentiation medium.
Challenges
Regeneration efficiency is generally lower in monocots than in dicots, requiring optimized culture conditions.
Direct Regeneration
Some plants (such as A. thaliana) can be regenerated directly through somatic embryogenesis, eliminating the need for a callus stage.
Troubleshooting Plant Transformation Protocols
- Low Transformation Efficiency: This may be caused by improper explant selection, insufficient Agrobacterium activity, or poor co-cultivation conditions. This can be addressed by optimizing the explant type, increasing the Agrobacterium inoculum size, or extending the co-cultivation time.
- Failed Gene Integration: This may be due to missing T-DNA border sequences or poor vector stability. Strategies such as verifying the vector construction and using high-fidelity enzymes for PCR amplification can be employed.
- Non-resistance Phenotype: Check for correct insertion of the selectable marker gene or adjust the culture medium composition (e.g., increase antibiotic concentration).
- Regeneration Failure: This may be caused by an imbalance in hormone ratios (e.g., cytokinin/auxin ratio). Adjust the culture medium formulation (e.g., add BAP and NAA) or optimize the hormone ratio.
Methods for Improving Plant Transformation Efficiency
Vector Optimization
- Enhanced Promoters: Use strong promoters such as 35S and LAT52 to drive gene expression.
- Binary Vector System: Improve integration efficiency by utilizing double-ended T-DNA border sequences.
Tissue Selection
- Young Tissues: Transformation rates of immature embryos and callus are significantly higher than those of mature tissues.
- Open Ovary: The Arabidopsis CRABS-CLAW mutant maintains an open ovary, resulting in a 6-fold increase in transformation efficiency.
Physical/Chemical Assisted Methods
- Electroporation: Uses electric pulses to enhance cell membrane permeability, suitable for difficult-to-transform cells.
- PEG Concentration Gradient: Optimize the PEG concentration (e.g., 0.5-1%) through multiple rounds of selection.
Transient transformation techniques
- Microinjection: Directly injecting DNA into the cell nucleus, suitable for single-cell manipulation.
- Combined gene editing: The CRISPR-Cas9 system enables precise gene insertion/deletion, minimizing random integration.
Tools and Reagents
Plant Transformation Tools and Reagents
Transformation Reagents
- Chemical Reagents: Such as PEG for protoplast transformation and Silwet L-77 (surfactant) for the floral dip method.
- Selection Reagents: Such as kanamycin for selecting resistant plants and chloramphenicol for detecting gene expression.
- Washing Solutions: Used to terminate the transformation reaction and remove residual reagents, such as K wash solution.
Vector Construction Tools
- Agrobacterium vectors: Ti plasmids carrying T-DNA (such as pBI121) are used to achieve transformation by deleting or replacing hormone genes and inserting target genes.
- Gene Editing Vectors: Such as the guide RNA (gRNA) and Cas9 protein in the CRISPR-Cas9 system, used for targeted gene modification.
- Viral Vectors: Such as CMV or tobacco mosaic virus (TMV) can be used for transient expression and are suitable for rapid functional verification.
- Physical/Chemical Carriers: Such as gold/tungsten microparticles (particle bombardment) or PEG chemical transformation methods, suitable for protoplast or somatic cell transformation.
Transformation Equipment
- Electroporator: Transiently perforates the cell membrane using high-voltage pulses (e.g., 1000-2000 V/cm), enabling DNA delivery.
- Gene Gun: Uses high-pressure helium to eject DNA-encapsulated gold particles into cells at high speed.
- Agrobacterium Culture System: Requires maintaining a specific pH (e.g., 5.5-6.0) and hormone (e.g., IAA, NAA) concentrations to promote infection.
Selectable Markers and Reporter Genes
Selectable Markers
- Antibiotic resistance: Such as kanamycin (KanMX) and hygromycin (Hygromycin B), allowing for selection of transformed cells.
- Herbicide resistance: Such as the glyphosate resistance gene (BAR) or phosphonate resistance gene (PAT), suitable for resistance screening.
Reporter Genes
- Fluorescent Proteins, such as GFP (green fluorescent protein) and RFP (red fluorescent protein), are used to monitor gene expression in real time.
- Enzyme Activity Reporter Genes, such as β-glucuronidase (GUS), detect expression through substrate colorimetry.
Integration of Plant Transformation Technology and Biotechnology
Genome Editing Technology
Genome editing technology achieves efficient genetic improvement by precisely modifying plant genomes.
Targeted Nucleases
- Zinc Finger Nucleases (ZFNs) and Transcription Activator-Like Effector Nucleases (TALENs): These target specific sites through protein-nucleic acid interactions, but their design is complex and costly.
- CRISPR-Cas System: The Cas9 protein binds to a guide RNA (gRNA). The gRNA guides the Cas9 protein to the target sequence and cuts the DNA, creating a double-strand break. Gene editing is then achieved through non-homologous end joining (NHEJ) or homology-directed repair (HDR). CRISPR-based gene editing tools, such as Cas9, have become mainstream due to their efficiency and ease of use.
Application Examples
- Disease Resistance Breeding: Enhance crop resistance by knocking out pathogen-related genes using CRISPR.
- Quality Improvement: Targeted editing of starch synthesis genes (e.g., Waxy) to improve rice amylose content.
- Marker Removal: Removal of exogenous marker genes using HDR or NHEJ strategies.
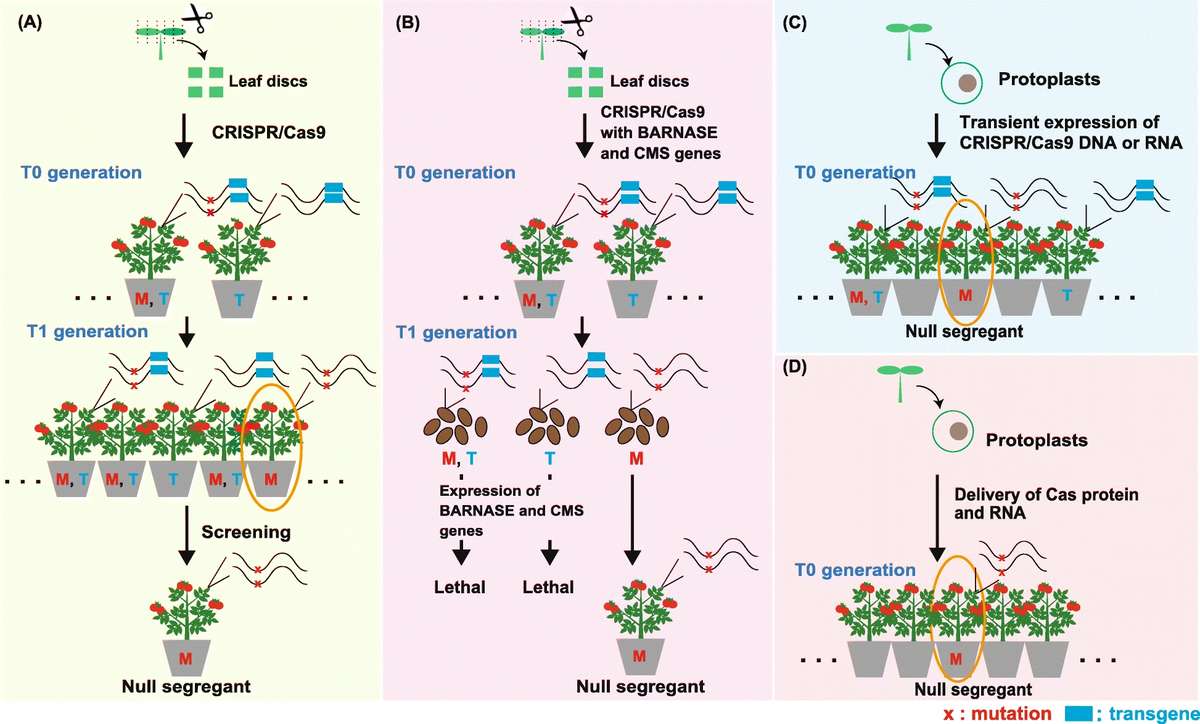 Fig. 2. Generation of null segregants in plants by CRISPR/Cas9 technology. (Wada, et al. 2020)
Fig. 2. Generation of null segregants in plants by CRISPR/Cas9 technology. (Wada, et al. 2020)
Synthetic Biology
Synthetic biology constructs complex metabolic pathways or regulatory networks through the design and assembly of biological components.
Microalgae Engineering
Chlamydomonas reinhardtii, a model photosynthetic eukaryote, is used to produce biofuels or pharmaceuticals through chloroplast genome engineering.
Using marker genes such as aadA to screen chloroplast transformants and develop multigene co-expression systems.
Plant Metabolic Engineering
Expressing heterologous metabolic pathways (e.g., synthesizing terpenoids) in rice chloroplasts to improve yield.
Optimizing photosynthesis efficiency and enhancing carbon fixation capacity through synthetic biology.
Commercial Applications
Plant transformation technology has a wide range of applications in agriculture, primarily including:
- Resistance Improvement: By introducing genes that confer resistance to herbicides, pests and diseases, or stresses (such as drought and salinity), crops can improve their environmental adaptability and yield. For example, herbicide-resistant soybeans and insect-resistant cotton are already commercially cultivated.
- Nutritional Fortification: Biofortification is used to increase the nutritional content of crops, including vitamins and minerals, such as beta-carotene-rich Golden Rice.
- Industrial Applications: Plants can be used to produce biofuels (such as ethanol), bioplastics, or pharmaceutical compounds (such as artemisinin).
- Basic Research and Breeding: Transformation technologies are used to analyze gene function and accelerate molecular breeding processes, such as using CRISPR-Cas9 technology to precisely edit crop genomes to improve agronomic traits.
Commonly Used Transgenic Plants and Their Characteristics
- Dicots: Arabidopsis, tobacco, tomato, etc., are often used as research models due to their high transformation efficiency and strong regeneration capacity.
- Monocots: Cereal crops such as rice, maize, and wheat have been commercialized using Agrobacterium-mediated transformation, but transformation is relatively challenging.
- Cash crops: Insect-resistant cotton, herbicide-tolerant soybeans, and virus-resistant papaya, etc., have been precisely modified using gene editing technologies (such as CRISPR-Cas9).
Model Plants and Successful Case Studies
Plant Selection in Plant Transformation
Selecting model plants requires comprehensive consideration of the following factors:
- Transformation Efficiency: For example, the floral dip method for Arabidopsis can achieve a transformation rate of over 50%, while rice transformation requires gene guns or Agrobacterium.
- Regeneration Ability: Tobacco leaves have strong regeneration capacity, making them suitable for transformation via the leaf disc method; Arabidopsis can be genetically transformed through seed regeneration.
- Genomic Information: The genomes of Arabidopsis and rice have been fully sequenced, facilitating the design of precise transformation strategies.
- Ecological Relevance: Selecting model plants closely related to the target crop (such as Arabidopsis and cruciferous crops) can improve transformation efficiency.
Model Plants in Transformation Research
- Arabidopsis: Due to its short life cycle and the availability of whole-genome sequencing data, Arabidopsis has become a preferred model for plant transformation research. Arabidopsis can be efficiently transformed using the floral dip method, generating transgenic progeny without the need for tissue culture. This method is often used for positional cloning (e.g., screening for genes through T-DNA insertional mutagenesis).
- Tobacco: The leaf disc method allows for rapid callus induction and plant regeneration, making it commonly used for gene function verification.
- Rice: Agrobacterium-mediated transformation or gene gun techniques are used to develop disease-resistant and drought-tolerant varieties.
Case Studies of Plant Transformation in Agriculture
- Insect-Resistant Cotton: The Bt gene (derived from Bacillus thuringiensis) is introduced into cotton, significantly reducing insect pest incidence and pesticide use.
- Herbicide-Tolerant Soybeans: Through Agrobacterium-mediated transformation, the EPSPS gene is introduced into soybeans, conferring resistance to the herbicide glyphosate.
- Golden Rice: Through multi-step genetic engineering, the zeaxanthin synthase gene (psy) and genes related to carotene synthesis are introduced into rice to increase vitamin A content.
 Fig. 3. Polished white and Golden Rice and (a different cultivar, after 2 months of postharvest storage) after cooking. (Dobock, 2019)
Fig. 3. Polished white and Golden Rice and (a different cultivar, after 2 months of postharvest storage) after cooking. (Dobock, 2019)
Comprehensive Evaluation and Challenges
Advantages and Challenges of Plant Transformation
Advantages
- High Efficiency: Agrobacterium-mediated transformation is highly efficient in most plants. For example, transformation rates in Arabidopsis can exceed 90%.
- Stability: Stably integrated transgenic plants can be obtained through tissue culture and regeneration techniques.
- Versatility: Suitable for a variety of scenarios, including gene editing, functional gene screening, and metabolic pathway modification.
Challenges
- Low Transformation Efficiency: Transformation of some crops (such as corn and wheat) is difficult, requiring optimized methods (such as gene guns and protoplast transformation).
- Randomness of Insertion Sites: Random integration of exogenous genes may lead to genomic instability or phenotypic variation.
- Cost and Time: Large-scale production of transgenic plants requires significant manpower and resources, and the screening process is time-consuming.
Impacts of plant transformation on the environment
- Potential Risks: Transgenic crops may spread genes to wild populations through pollen, causing ecological imbalances.
- Sustainability: Herbicide-tolerant crops may lead to the evolution of weed resistance, increasing pesticide use pressure.
- Biodiversity Conservation: Transgenic technology may reduce reliance on traditional breeding, but a balance must be struck between economic benefits and ecological safety.
Ethical and legal issues of plant transformation
- Ethical Controversy: Public acceptance of transgenic crops is divided, and some countries (such as the European Union) implement strict regulations on genetically modified foods.
- Intellectual Property: The patent ownership of transgenic seeds has led to legal disputes.
- Policies and Regulations: The approval process for transgenic crops varies significantly across countries, and the establishment of unified standards is needed to promote technology dissemination.
Plant Transformation Services
At Lifeasible, we use a variety of methods to provide high-quality plant transformation services, including Agrobacterium-mediated transformation, PEG-mediated transformation, gene gun, and virus-mediated transformation. Our capabilities cover model plants, food crops, cash crops, vegetables, fruits, and more. Discover our plant transformation services.
Frequently Asked Questions (FAQs)
Q: What is plant transformation technology?
A: Plant transformation technology refers to the introduction of foreign genes into the plant genome, thereby modifying its genetic traits. It is used for crop improvement and biotechnology advancements.
Q: What is plant transformation?
A: Plant transformation involves transferring a target gene into a plant through physical, chemical, or biological methods, and ensuring its stable expression, thereby conferring new traits on the plant.
Q: What is the difference between stable and transient transformation in plant transformation?
A: Stable transformation involves the integration of a foreign gene into the plant genome, making it heritable by future generations. Transient transformation involves temporary expression of the foreign gene and does not involve genomic integration.
Q: What are the common methods used in plant transformation?
A: Common methods include Agrobacterium-mediated transformation, biolistics, viral-mediated transformation, and PEG-mediated transformation.
Q: How can plant transformation efficiency be optimized?
A: Transformation efficiency can be improved by optimizing plasmid vectors, selecting young tissues, using chemical/physical aids such as electroporation, and adjusting culture conditions.
Q: What are the practical applications of plant transformation technology?
A: Practical applications include improving plant resistance, nutritional fortification, and industrial production of biofuels, bioplastics, or pharmaceutical compounds.
Q: What are the main challenges in plant transformation?
A: Major challenges include low transformation efficiency in some crops, instability caused by random integration of foreign genes, and the high cost and time required to produce large transgenic plants.
Q: What ethical and environmental issues does plant transformation technology face?
A: There are potential ecological risks, such as the spread of transgenes to wild populations through pollen, as well as differing public acceptance of transgenic crops and legal patent issues.
References
- Su, W., et al. (2023). Technological development and application of plant genetic transformation. International Journal of Molecular Sciences, 24(13), 10646. DOI: 10.3390/ijms241310646.
- Gao, C. (2021). Genome engineering for crop improvement and future agriculture. Cell, 184(6), 1621-1635. DOI: 10.1016/j.cell.2021.01.005.
- Koul, B. (2022). Plant Transformation Techniques. In: Cisgenics and Transgenics. Springer, Singapore. DOI: 10.1007/978-981-19-2119-3_1.
- Quezada, G.D.Á., Ijaz, S., Malik, R. (2024). Genetic Transformation in Plants: Methods and Applications. In: Ijaz, S., Ul Haq, I., Mohamed Ali, H. (eds) Trends in Plant Biotechnology. Springer, Singapore. DOI: 10.1007/978-981-97-0814-7_2.
- Newell, C. A. (2000). Plant transformation technology: developments and applications. Molecular biotechnology, 16(1), 53-65. DOI: 10.1385/MB:16:1:53.
- Low, L. Y., et al. (2018). Transgenic plants: gene constructs, vector and transformation method. In New visions in plant science. intechopen. DOI: 10.5772/intechopen.79369.
- Parrott, W., et al. (2024). Overcoming Barriers in Plant Transformation: A Focus on Bioenergy Crops (No. DOE/SC-0215). US Department of Energy (USDOE), Washington, DC (United States). Office of Science. DOI: 10.2172/2335710.
- Xu, H., et al. (2022). Progress in soybean genetic transformation over the last decade. Frontiers in plant science, 13, 900318. DOI: 10.3389/fpls.2022.900318.
- Wada, N., et al. (2020). Precision genome editing in plants: state-of-the-art in CRISPR/Cas9-based genome engineering. BMC Plant Biology, 20(1), 234. DOI: 10.1186/s12870-020-02385-5.
- Dobock, A. (2019). Golden Rice: To Combat Vitamin A Deficiency for Public Health, Vitamin A. DOI: 10.5772/intechopen.84445.
Related Services
 Fig. 1. General procedure of Agrobacterium-mediated and biolistic soybean transformation. (Xu, et al. 2022)
Fig. 1. General procedure of Agrobacterium-mediated and biolistic soybean transformation. (Xu, et al. 2022) Fig. 2. Generation of null segregants in plants by CRISPR/Cas9 technology. (Wada, et al. 2020)
Fig. 2. Generation of null segregants in plants by CRISPR/Cas9 technology. (Wada, et al. 2020) Fig. 3. Polished white and Golden Rice and (a different cultivar, after 2 months of postharvest storage) after cooking. (Dobock, 2019)
Fig. 3. Polished white and Golden Rice and (a different cultivar, after 2 months of postharvest storage) after cooking. (Dobock, 2019)