Plant transformation, a core foundation of modern plant biotechnology, has provided revolutionary tools for the advancement of agriculture and biological sciences. Since the development of Agrobacterium tumefaciens-mediated transformation in the 1980s, plant transformation technology has rapidly evolved, becoming a key tool for gene function research, crop improvement, and bioreactor development. Unlike traditional breeding, which relies on natural variation and hybridization, plant transformation allows for direct manipulation of genetic material, resulting in targeted improvements in yield, stress tolerance, and nutritional value.
Understanding the molecular biology underlying transformation is crucial. Behind every successful transgenic plant lies a complex series of cellular events: the recombination of foreign DNA into the host genome, the regulation of transgene expression at the transcriptional and translational levels, and the molecular markers used to track, validate, and optimize this process. Each of these layers sheds light on why some transformation events succeed and why others fail, and how researchers can improve their efficiency and robustness.
This article explores the molecular basis of plant transformation, focusing on DNA recombination, transgene expression, and molecular markers. These processes not only underpin academic research in functional genomics but also drive the industrial application of plant biotechnology in agriculture, synthetic biology, and pharmaceutical production.
DNA recombination is the core mechanism for the stable integration of exogenous genes in plant transformation. According to molecular biology principles, DNA recombination occurs primarily through two pathways: homologous recombination (HR) and non-homologous end joining (NHEJ).
HR is the most fundamental mechanism of DNA recombination, achieving genetic recombination through nucleotide exchange between homologous sequences. The process involves DNA breakage, strand exchange, and repair, ultimately producing homologous recombination products. In plants, HR relies on the coordinated action of enzymes such as the RecBCD complex, RecA protein, and RuvC protein. However, the low incidence of spontaneous homologous recombination in plants limits its application in gene editing.
NHEJ is a nonspecific repair mechanism that repairs broken DNA ends by directly joining them. This process relies on enzymes such as DNA-PK and Artemis, but is inherently stochastic, potentially leading to insertions and deletions and lacking precision. In plant genomes, NHEJ often leads to nonspecific integration, affecting gene stability.
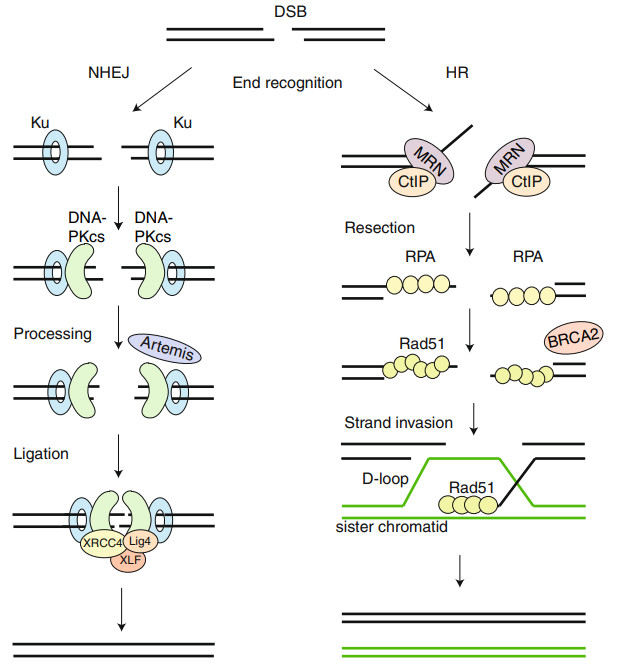 Fig. 1. HR and NHEJ. (Brandsma, et al. 2012)
Fig. 1. HR and NHEJ. (Brandsma, et al. 2012)
The role of DNA recombination in plant transformation is primarily reflected in the stable integration and expression of exogenous genes. Taking Agrobacterium-mediated transformation as an example, T-DNA (a DNA fragment from a Ti plasmid) is integrated into the plant genome through homologous recombination, achieving stable expression of the exogenous gene. T-DNA integration relies on the plant cell's DNA repair mechanism and occurs primarily through nonhomologous recombination. This integration produces stable transformants whose offspring inherit the transgene.
Other transformation methods, such as gene gun-mediated transformation, also rely on DNA recombination mechanisms, introducing exogenous DNA into plant cells through physical or chemical means to achieve genomic integration.
In recent years, tools such as the CRISPR/Cas system and site-specific recombinases have significantly improved the precision and efficiency of DNA recombination. CRISPR/Cas systems (such as CRISPR/Cas9) use guide RNA to target specific DNA sequences, induce double-strand breaks, and combine with homologous recombination donor DNA to achieve precise gene editing. This technology has been widely used in plant genome editing, for example, through CRISPR/Cas9-mediated gene insertion, deletion, or point mutation, achieving precise genetic improvement.
Site-specific recombinase systems (such as the Cre/loxP and FLP/FRT systems) enable targeted excision or recombination at predetermined sites. For example, the Cre-loxP system can be used for single-copy integration or gene deletion in plant genomes, reducing nonspecific recombination.
The combined application of these tools has significantly improved the efficiency and precision of plant genome editing, advancing the development of plant biotechnology.
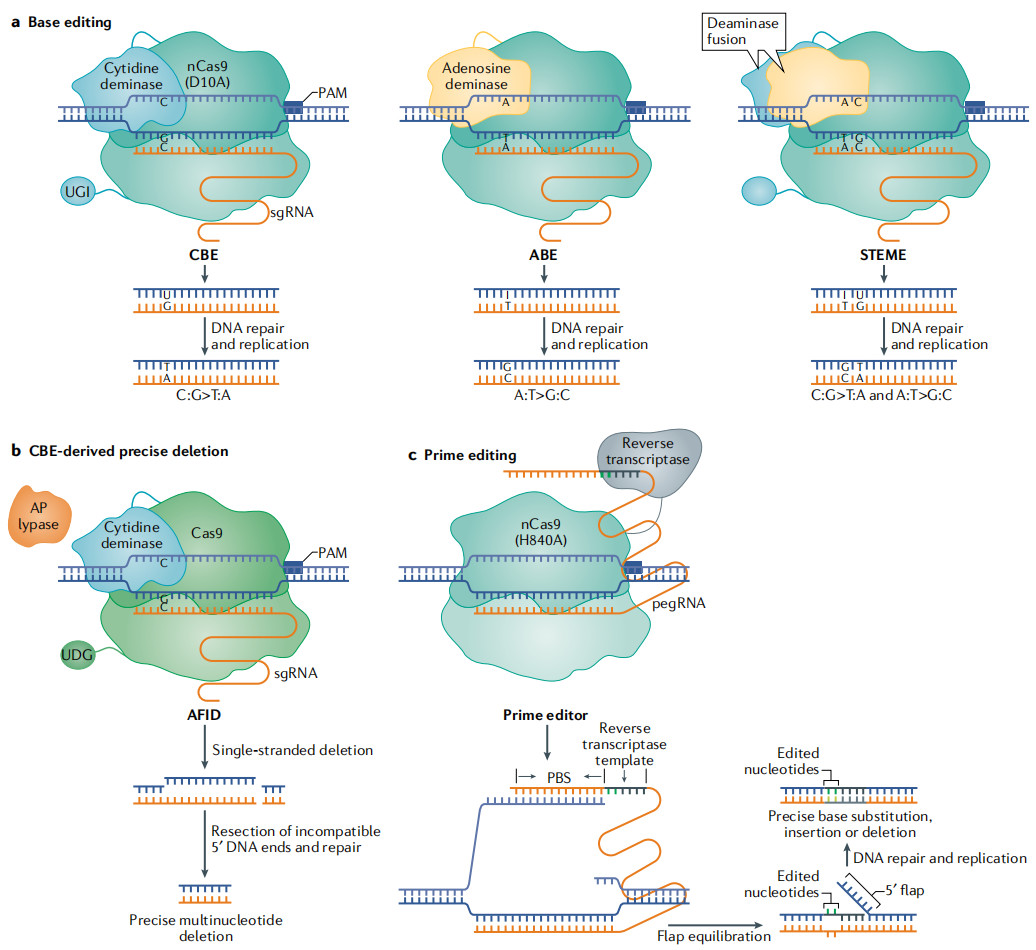 Fig.2. Deaminase-mediated and reverse transcriptase-mediated precise genome editing technologies in plants. (Zhu, et al. 2020)
Fig.2. Deaminase-mediated and reverse transcriptase-mediated precise genome editing technologies in plants. (Zhu, et al. 2020)
Once a transgene is integrated, its impact depends on its ability to be effectively expressed. Transgene expression involves multiple molecular processes: transcription, RNA processing, translation, and protein stability.
The strength and specificity of expression depend on the design of the transgene expression cassette. Common elements include:
Epigenetic regulation (e.g., DNA methylation and histone modifications) can influence the stability of gene expression. DNA methylation can lead to gene silencing, while RNA interference (RNAi) achieves gene silencing by degrading mRNA through small RNAs (e.g., siRNAs). For example, miRNA-mediated gene silencing is widely used in resistance research. In addition, post-transcriptional modifications (such as RNA editing and mRNA stability regulation) further influence the dynamics of gene expression.
Protein-level regulation involves codon optimization, fusion tags, and subcellular targeting signals. Codon optimization improves translation efficiency, fusion tags (such as His tags) facilitate protein purification, detection, or enhance solubility, and subcellular targeting signals (such as chloroplast targeting signals) direct protein localization to specific organelles, such as chloroplasts, vacuoles, or apoplasts, to achieve desired activity. Post-translational modifications (such as phosphorylation and glycosylation) further regulate protein function.
Successful examples of transgenic expression include:
Together, these examples demonstrate how molecular-level regulation can shape phenotype.
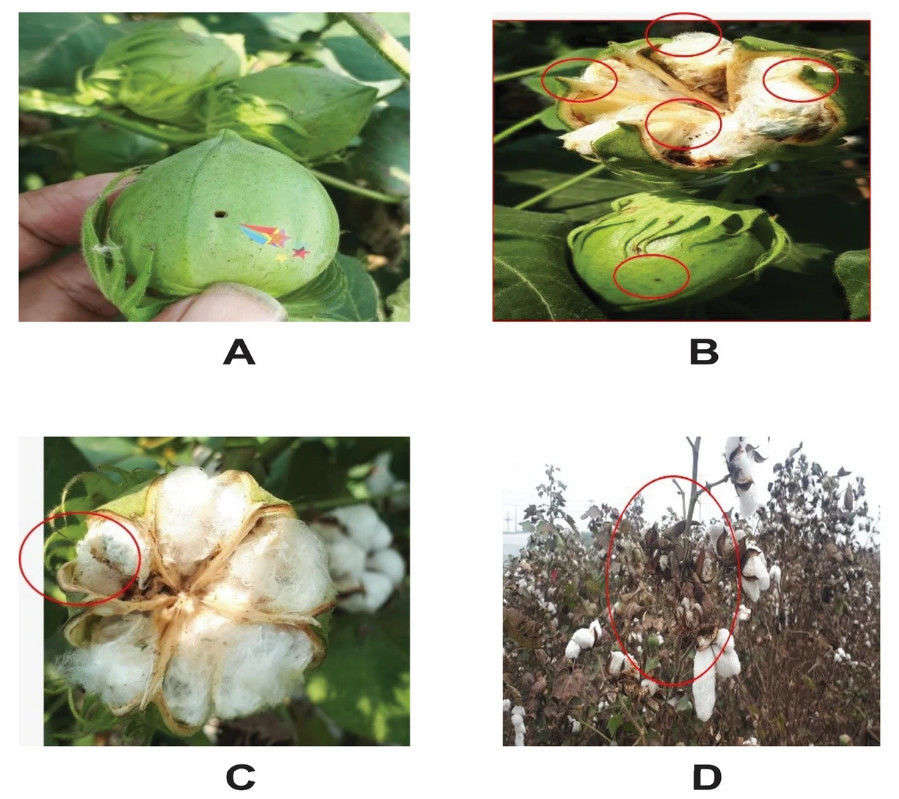 Fig. 3. Pink bollworm mortality assay in transgenic and non-transgenic cotton plants. Non-transgenic cotton plant leaf almost completely infected by pink bollworm. (Zafar, et al. 2022)
Fig. 3. Pink bollworm mortality assay in transgenic and non-transgenic cotton plants. Non-transgenic cotton plant leaf almost completely infected by pink bollworm. (Zafar, et al. 2022)
Molecular marker technology plays a key role in plant transformation. From marker selection to assisted breeding and marker-free transformation, it has promoted plant genetic improvement and sustainable agricultural development.
Molecular marker technology is primarily used in plant transformation to identify successful integration events and track transgene inheritance in progeny. By detecting polymorphisms in specific DNA sequences, molecular markers can verify the successful integration of a transgene into the host genome and track its genetic stability in subsequent generations. For example, PCR-based markers and fluorescent/visible markers can directly detect the expression or integration status of the target gene. Molecular markers can also be used to screen for transformation events, improving transformation and breeding efficiency.
Molecular marker technology encompasses various types, including:
MAS improves the efficiency of screening for target traits through marker-assisted breeding processes.
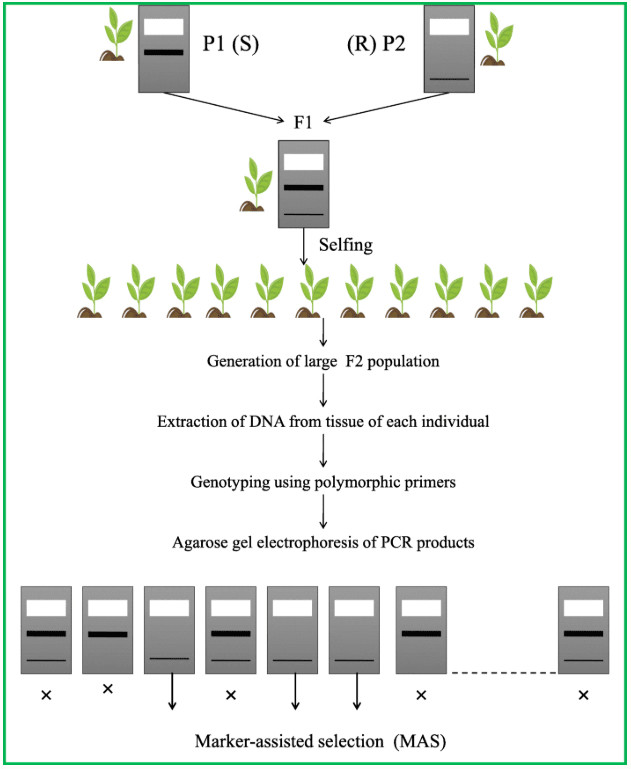 Fig. 4. The basic procedure of marker-assisted selection. (Hasan, et al. 2021)
Fig. 4. The basic procedure of marker-assisted selection. (Hasan, et al. 2021)
Marker-free strategies are gaining increasing attention to address biosafety and regulatory issues. These methods include:
Marker-free plants are more readily accepted by regulators and consumers while still benefiting from early-stage molecular marker-assisted verification.
Plant transformation is not a single step but rather an interconnected molecular process.
From a systems biology perspective, recombinant DNA, gene expression, and marker technologies together constitute a coordinated molecular process. This process involves the intersection of multiple disciplines, including molecular biology, bioinformatics, and systems biology, to achieve precise control of plant traits.
The molecular insights described above can be directly translated into practical applications:
In each case, understanding the molecular basis of transformation improves efficiency, reduces risk, and accelerates product development.
Despite significant advances in genetic engineering and molecular marker technologies, numerous challenges remain:
Future breakthroughs in plant transformation are likely to occur through multiple developments:
The future of plant transformation lies in making molecular processes predictable, precise, and universally applicable across species.
The molecular biology foundation of plant transformation consists of three pillars: DNA recombination, transgene expression, and molecular marker technology. Together, these processes determine transformation success and its impact on agriculture and biotechnology. As tools evolve from random integration to precise, marker-free, and regulated systems, plant transformation will continue to drive breakthroughs in crop improvement, synthetic biology, and biomanufacturing.
Q: What is plant transformation? Why is it important?
A: Plant transformation refers to the process of introducing new genetic material into the plant genome. It is crucial because it allows for the direct manipulation of inherited traits, thereby improving crop yield, stress tolerance, and nutritional content beyond what is achievable through traditional breeding.
Q: How does DNA recombination contribute to plant transformation?
A: DNA recombination facilitates the integration of new genes into the plant genome through homologous recombination (HR) or nonhomologous end joining (NHEJ). This integration is crucial for the stable expression of transgenes.
Q: What are molecular markers? How are they used in plant transformation?
A: Molecular markers are sequences used to track the inheritance of plant genes. In plant transformation, they can identify successful integration events and track the transgenerational stability of transgenes.
Q: What role does CRISPR/Cas technology play in plant transformation?
A: CRISPR/Cas technology enables precise editing of plant genomes by targeting specific DNA sequences, enabling the precise insertion or deletion of genes, thereby increasing the efficiency of plant genetic engineering.
Q: Why do some transgenes become silent over time?
A: If a transgene integrates into heterochromatin, DNA methylation, RNA interference pathways, or position effects can lead to transgene silencing. Construct design and insulator sequences can help mitigate this.
Q: How does transgene silencing impact plant genetic engineering?
A: Transgene silencing can lead to reduced or lost expression of the introduced trait, potentially rendering the engineered trait ineffective and negating the benefits of genetic engineering.
Q: What is a marker-free transformation strategy?
A: Marker-free transformation omits the use of a selectable marker in the final plant product to address biosafety and consumer concerns. Marker removal is typically achieved using techniques such as negative selection markers or recombinase systems.
Q: What is the importance of tissue-specific promoters in transgene expression?
A: Tissue-specific promoters restrict gene expression to certain parts of the plant, thereby enhancing trait control and efficiency. For example, expression of a resistance trait can be restricted to tissues susceptible to a specific stress.
Q: Why is understanding the molecular basis of transformation crucial for biotechnology?
A: A deeper understanding of the molecular basis can ensure greater efficiency and precision in the development of transgenic crops, which is crucial for applications in agriculture, synthetic biology, and pharmaceuticals.
References