Plant transformation has become one of the cornerstones of modern plant biology and agricultural biotechnology. By introducing and expressing foreign genes, transformation techniques provide powerful opportunities for studying gene function, modifying desirable agronomic traits, and producing valuable biomolecules in plant systems. Recent decades have seen remarkable advances in transformation technologies, providing researchers with tools to address pressing global agricultural challenges, including food security, malnutrition, and the impacts of climate change.
In plant genetic engineering, stable transformation and transient transformation are the two main transformation strategies. While these two methods share the goal of manipulating the plant genome or gene activity, they differ in their underlying mechanisms, experimental timelines, modes of inheritance, and areas of application. Stable transformation involves the integration of foreign DNA into the host genome, allowing the introduced gene to be transmitted to subsequent generations. Transient transformation, on the other hand, results in short-term gene expression without permanent integration into the plant genome.
Understanding when and how to use these methods is crucial for both basic research and applied plant biotechnology. This article aims to systematically analyze the mechanisms, applications, and differences between stable and transient transformation, explore their strategic value in science and industry, and provide theoretical support and practical guidance for the development of plant genetic engineering.
Stable transformation and transient transformation are two core methods in plant genetic engineering. Their core differences lie in the method of exogenous DNA integration and inheritance.
Stable transformation occurs when exogenous DNA integrates into the host genome and is passed down through generations, achieving long-term expression and genetic stability. Its molecular mechanism involves DNA integration into chromosomal DNA, ensuring its inheritance across cell division and generations. Transformed plants are considered genetically modified organisms (GMOs) or, in the case of precise editing, gene-edited plants.
Stable transformation is typically mediated by Agrobacterium tumefaciens, biolistic bombardment, or the CRISPR/Cas system and enables long-term genetic improvement of crops.
Transient gene expression relies on the transient presence of exogenous DNA and does not involve genomic integration. The introduced DNA or RNA survives only for a limited time in plant cells and is gradually degraded or diluted during cell division. Transient gene expression enables short-term functional studies through temporary expression, such as gene function verification or rapid expression analysis. Its molecular mechanism relies on the transient presence of exogenous DNA, not integration, and is typically achieved through methods such as Agrobacterium infiltration, particle bombardment, or viral vectors.
Transient expression typically utilizes episomal vectors, Agrobacterium infiltration, viral replicons, or protoplast transfection, providing a rapid and flexible expression method that meets the needs of both experimental and industrial production.
The core of stable plant transformation lies in integrating foreign genes into the plant genome, achieving long-term, heritable expression. Its technical features include:
Crop Genetic Improvement
Functional Genomics
Constructing stable mutants or reporter gene lines to study gene function.
Industrial Applications
Stable transgenic plants can be used as biofactories to produce recombinant proteins, vaccines, and other biological products.
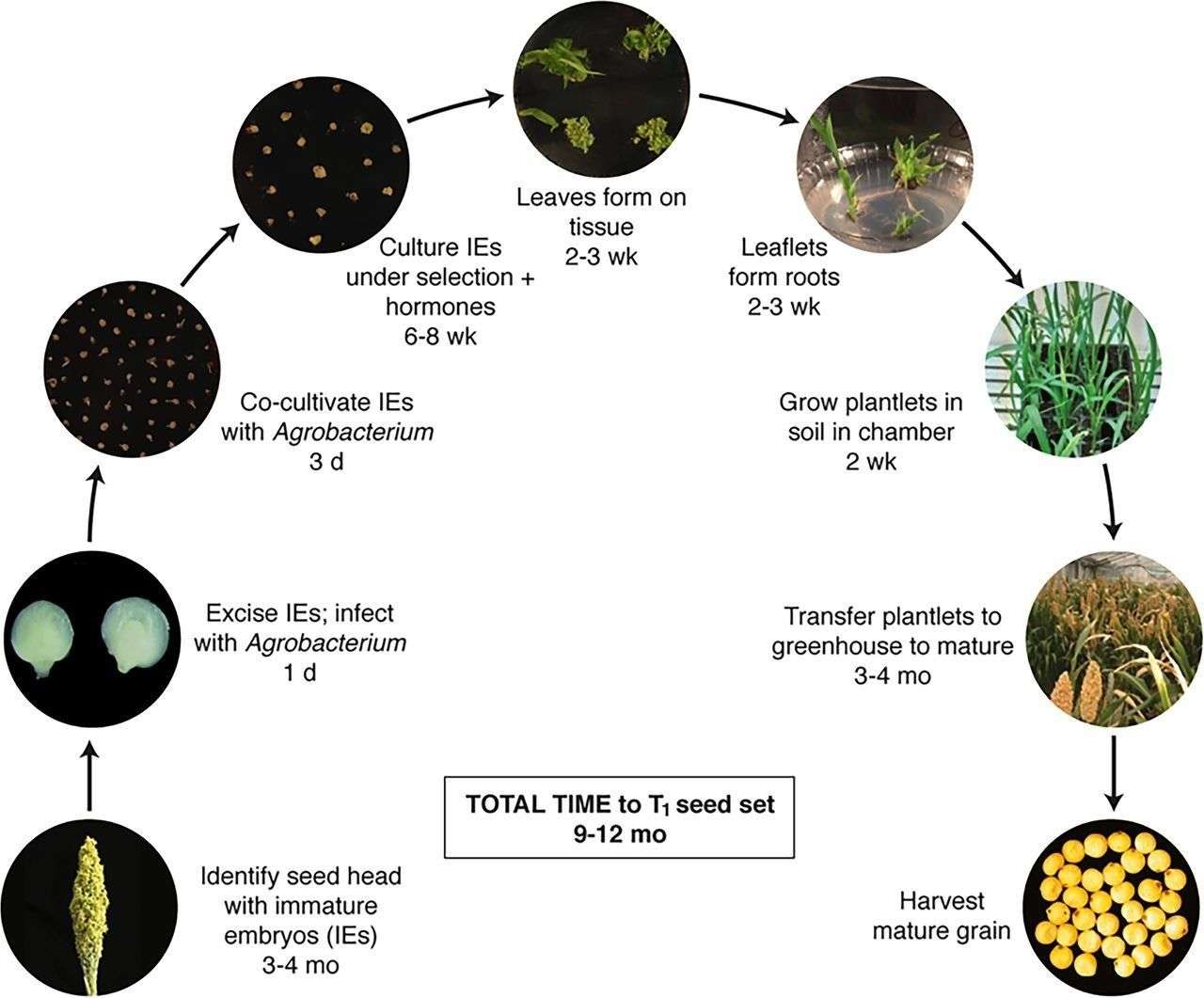 Fig. 1. Stable transformation process and timelines for sorghum. (Altpeter, et al. 2016)
Fig. 1. Stable transformation process and timelines for sorghum. (Altpeter, et al. 2016)
Transient gene expression technology achieves rapid expression of exogenous genes through a variety of delivery pathways. Common delivery methods include:
Reporter systems (such as GFP and GUS) can be used to monitor gene expression in real time. Transient expression typically lasts for days to weeks, and expression levels are independent of gene location and unaffected by gene silencing.
Transient expression offers significant advantages:
Transient expression has broad applications in multiple fields:
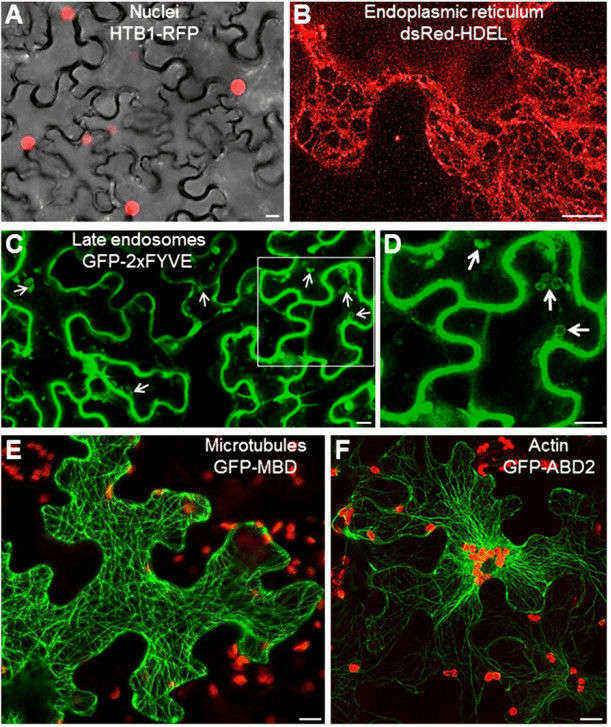 Fig. 2. Transient transformation of Nicotiana benthamiana leaves using A. tumefaciens with fluorescent organelar markers. (Krenek, et al. 2015)
Fig. 2. Transient transformation of Nicotiana benthamiana leaves using A. tumefaciens with fluorescent organelar markers. (Krenek, et al. 2015)
While transient expression offers significant advantages, it also has limitations:
When evaluating these methods, researchers and industry practitioners weigh the timeline, scalability, and goals of each approach.
| Feature | Stable Transformation | Transient Transformation |
| DNA integration | Integrated into genome | Episomal/temporary expression |
| Heritability | Passed to progeny | Not inherited |
| Timeline | Months–years | Days–weeks |
| Expression Level | Affected by position effects | Typically higher than stable lines |
| Expression Duration | Long-term stable (generations) | Short-term (typically 5–7 days) |
| Applications | Crop improvement, long-term trait expression, breeding, pharmaceuticals | Rapid gene validation, protein production, synthetic biology |
| Regulation | Subject to strict GMO oversight | Often exempt from GMO regulations |
| Scalability | Field-level agricultural deployment | Laboratory, greenhouse, bioreactor scale |
The choice depends on project goals. Transient expression is ideal for rapid testing or short-term production, while stable transformation is essential for durable trait improvement and commercial crop development.
Transient systems are widely used for proof-of-concept studies and recombinant protein production due to their rapid and efficient nature. For example, transient expression systems can rapidly achieve target gene expression for protein production, functional studies, and vaccine development. Furthermore, transient expression technologies (such as viral vector systems) can rapidly assess gene expression efficiency and optimize expression vector design. Transient systems are also suitable for gene function studies, such as gene silencing and knockout studies, because they do not require long-term stable transformation.
Stable transformation is suitable for long-term production needs and genetic improvement. For example, stable transformation can integrate foreign genes into the plant genome, establishing transgenic crop lines and achieving the inheritance and long-term expression of traits, enabling improved crop traits (such as disease resistance and yield enhancement) or metabolite production (such as biofuels and industrial enzymes).
The decision between using a stable or transient system is rarely absolute; many projects strategically combine the two. Transient expression can serve as a preliminary step to stable transformation, allowing for screening and optimization of expression systems. This complementary application highlights how transient expression systems can serve as a building block for stable transformation, accelerating the transition from research to market.
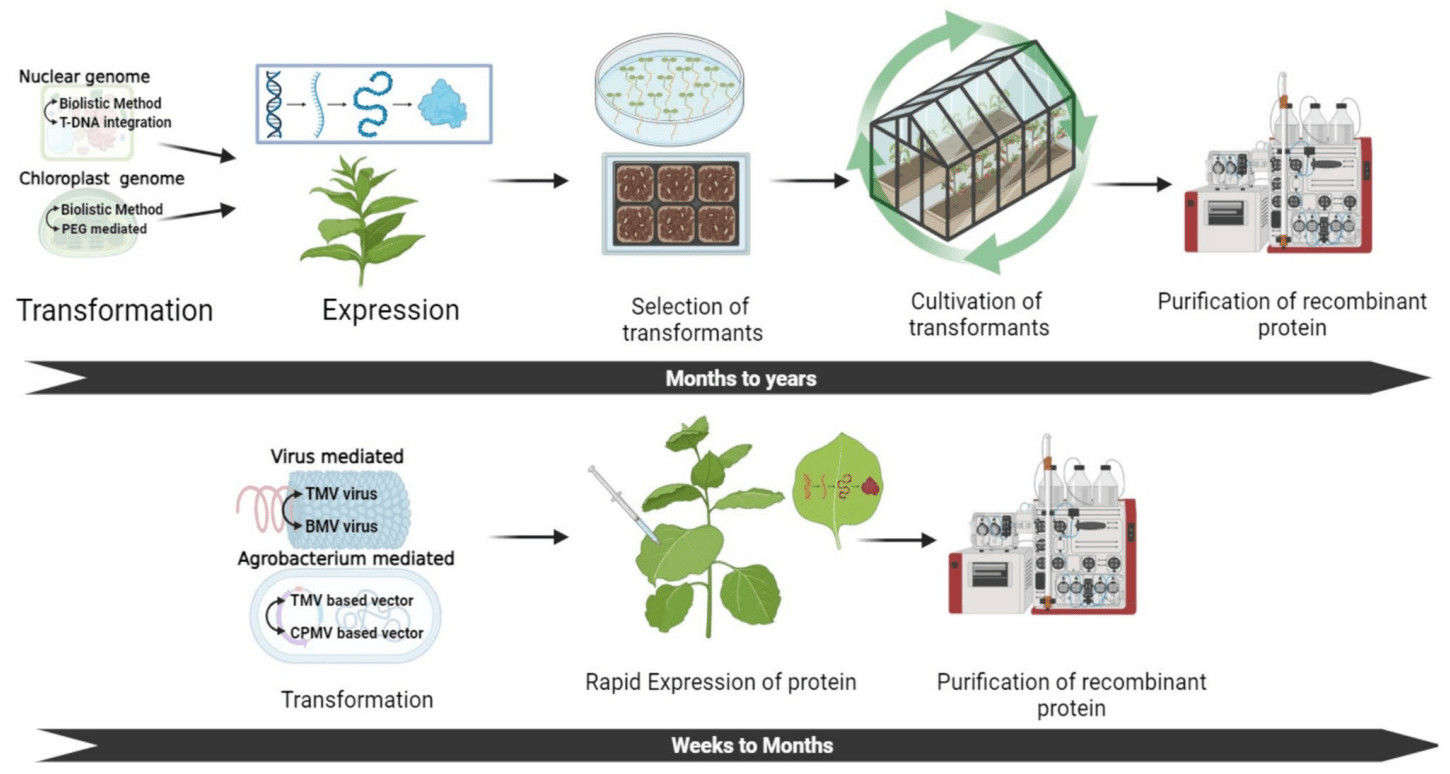 Fig. 3. Comparison of stable and transient expression systems for recombinant protein production in plant-based systems. (Jadhav, et al. 2024)
Fig. 3. Comparison of stable and transient expression systems for recombinant protein production in plant-based systems. (Jadhav, et al. 2024)
Despite the success of both transformation approaches, they still face challenges.
Looking forward, the integration of transformation technologies with automated tissue culture, machine learning for construct design, and synthetic promoter engineering is expected to expand the capabilities of both stable and transient transformation.
Stable and transient transformation represent two powerful and complementary approaches in plant biotechnology. Stable transformation is the foundation for long-term genetic improvement and commercial trait application, while transient expression offers unparalleled speed and flexibility for discovery and biomanufacturing. These approaches are not competing but form a continuum of strategies that enable researchers and industry to tailor translational approaches to their specific goals. Together, they are driving innovation in plant science and agriculture, fostering a more sustainable, efficient, and resilient food system for the future.
Lifeasible understands that plant biotechnology R&D requires flexibility, precision, and reliable results. Therefore, we offer a comprehensive range of stable transformation and transient gene expression services to meet the diverse needs of our partners.
Whether your focus is genetic improvement, crop enhancement, functional genomics, or industrial applications, our team of experts combines advanced transformation technologies with stringent quality standards to accelerate your project.
Contact our experts today to receive a personalized stable transformation or transient gene expression solution.
Q: What is the main difference between stable transformation and transient transformation?
A: Stable transformation involves the permanent integration of foreign DNA into the plant genome, making the trait heritable from generation to generation. Transient transformation results in temporary gene expression without genomic integration, typically lasting only a few days to weeks.
Q: When should stable transformation be chosen over transient expression?
A: Stable transformation is best suited for long-term projects, such as crop improvement, breeding programs, or any application requiring heritable traits. Transient expression is more suitable for rapid testing, protein production, or short-term functional studies.
Q: How long does each process take?
A: Stable transformation can take several months to over a year, depending on the plant species and the complexity of the project. Transient expression can produce results within days to weeks.
Q: Are products from transient expression considered GMOs?
A: In many regions, transient expression is not classified as GMOs because the DNA does not integrate into the genome. However, classification depends on the local regulatory framework.
Q: Can transient expression be used as a preparatory step before stable transformation?
A: Yes. Many researchers first test their gene constructs using transient expression systems before moving on to longer-term stable transformation. This strategy saves time and resources.
Q: Does Lifeasible offer customized solutions for specific plant species?
A: Of course. Our team specializes in optimizing transformation protocols for a wide range of plant species, including model plants, food crops, commercial crops, and more. We work closely with our clients to tailor our solutions to their specific needs.
References