Throughout the long process of biological evolution, the number of chromosomes (karyotype) of each species usually remains highly stable. Drastic changes in chromosome number or structure often lead to gamete lethality or severe developmental defects. However, with the revolutionary breakthroughs in gene editing technology, scientists have begun to explore whether it is possible to precisely rewrite the basic architecture of life—chromosomes—like writing a computer program.
On November 20, 2025, a team led by Holger Puchta from the Karlsruhe Institute of Technology published a landmark study in Science titled "CRISPR-Cas–mediated heritable chromosome fusions in Arabidopsis." They successfully used CRISPR-Cas gene editing technology to achieve heritable chromosome fusion in the model plant Arabidopsis thaliana, stably reducing its chromosome number from the natural 10 (2n=10) to 8 (2n=8). This research not only achieved targeted chromosome number reduction in plants for the first time but also revealed the surprising robustness of the plant genome in the face of large-scale artificial rearrangement, opening up new avenues for crop breeding and synthetic biology.
The research team did not perform random cutting, but instead designed a precise and controllable two-step editing strategy.
First, two double-strand DNA breaks were simultaneously induced in A. thaliana embryo cells, translocating and fusing the short arm of chromosome 3 to chromosome 1, resulting in an intermediate plant.
Subsequently, a second round of editing was performed on the intermediate plant to translocate the remaining long arm of chromosome 3 to another recipient chromosome (e.g., chromosome 5).
During this process, the "emptied" centromere of chromosome 3 and its two telomeres formed a small chromosome lacking essential genes. This useless fragment was naturally eliminated and lost during subsequent cell divisions, thus achieving a net reduction in the total number of chromosomes.
Through this strategy, the team successfully constructed two different eight-chromosome homozygous lines: the F8 line (both arms of chromosome 3 fused to chromosome 1) and the T8 line (both arms of chromosome 3 fused to chromosomes 1 and 5, respectively).
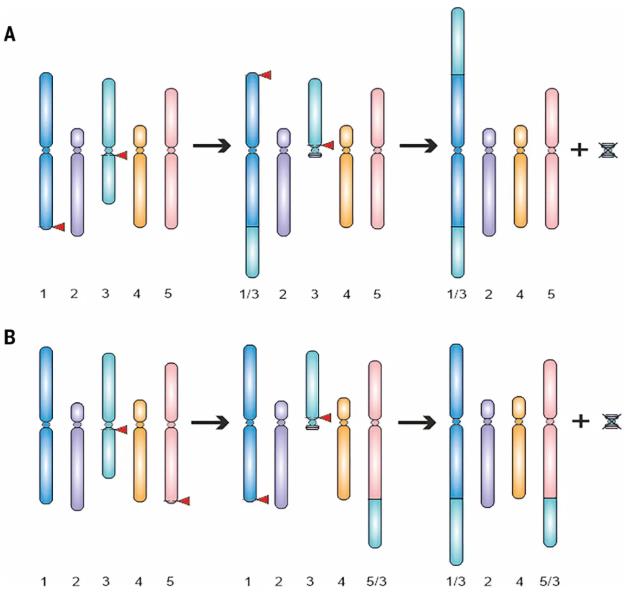
Figure 1. The generation of the two eight-chromosome lines F8 and T8. (Rönspies, et al. 2025)
The study definitively confirmed the occurrence of chromosome fusion events and a stable reduction in chromosome number (only 8 centromere signals were detected) using techniques such as fluorescence in situ hybridization, high-throughput sequencing, and centromere protein CENH3 immunostaining.
The most surprising finding was that these plants, which had undergone major genomic restructuring, showed almost no difference in growth and development compared to the wild type:
Another key finding of the study lies in its reproductive biology consequences, which directly point to immense application potential.
This research transcends the traditional scope of gene editing, achieving a leap from editing genes to designing chromosomes. It demonstrates that through precise CRISPR-Cas engineering, we can safely and heritably alter the number and structure of plant chromosomes without compromising their normal life activities.
This work marks the beginning of the era of "targeted karyotype engineering." Its implications are far-reaching:
In the future, applying this technological system to major crops such as rice and wheat is expected to give rise to a completely new breeding paradigm, providing a new technological weapon to address global food security challenges. From 10 to 8, it's not just a change in numbers, but a profound leap in humanity's ability to understand and manipulate the code of life.