On November 20, 2025, a team led by Stanton B. Gelvin from Purdue University, along with their collaborators, published a research paper titled "Myosin VIII and XI isoforms interact with Agrobacterium VirE2 protein and help direct transport from the plasma membrane to the perinuclear region during plant transformation" in Plant Communications. This study reveals the regulatory mechanism of two types of plant myosins (myosin VIII and XI) in the anchoring and intracellular transport of the key virulence effector protein VirE2 during Agrobacterium-mediated plant genetic transformation, providing a new perspective for a deeper understanding of the molecular mechanisms of Agrobacterium-plant interaction.
Agrobacterium tumefaciens is a soilborne pathogen that naturally transforms plant cells, and its mediated genetic transformation technology is one of the most crucial tools in plant genetic engineering and gene function research. During infection, Agrobacterium not only transfers the T-DNA single strand (T-strand) into plant cells but also simultaneously delivers various virulence effector proteins. Among them, VirE2 protein is the most abundant and crucial for transformation efficiency. Previous studies have shown that VirE2 binds to the T-strand in the plant cytoplasm, forming a protected T-complex, and assists its entry into the nucleus. However, how VirE2 itself is transported to the perinuclear region, and which components of the host cell are involved in this process, remain unclear. Myosins, as molecular motors that move along actin filaments, are key executors of intracellular material transport, but their specific function in Agrobacterium transformation remains to be elucidated.
To investigate the role of myosin in Agrobacterium-mediated transformation, researchers first examined the transformation sensitivity of various Arabidopsis actin and myosin mutants. The study found that the transient and stable transformation efficiency in the roots of Arabidopsis actin gene act2 and act7 mutants was significantly reduced by 3-5 times compared to the wild type, and this transformation sensitivity could be restored by introducing wild-type ACT7 cDNA. This indicates that actin expressed in the roots is essential for successful Agrobacterium-mediated transformation. Among numerous single myosin mutants, only the myosin XI-h mutant showed a significant transformation defect. However, when multiple myosin genes were mutated simultaneously, the transformation efficiency decreased significantly, suggesting functional redundancy among members of the myosin family. Although previous studies indicated that myosin XI-K is crucial for VirE2 transport, this study found that a single myosin XI-k mutation did not affect transformation efficiency, suggesting possible functional compensation by other myosin XI subtypes in Arabidopsis.
To clarify the specific functions of different myosin VIII members, researchers separately complemented the myosin VIII-1/2/a/b quadruple mutant with cDNAs of each myosin VIII. The results showed that expressing myosin VIII-2, VIII-A, or VIII-B cDNA could partially restore the transformation sensitivity of the quadruple mutant, while expressing myosin VIII-1 cDNA had no such effect. Overexpression of myosin VIII-1 in wild-type Arabidopsis actually enhanced transformation, indicating that although VIII-1 has a different function, it is also a limiting factor in the transformation process.
Using various techniques including yeast two-hybrid, in vitro pull-down, in vivo co-immunoprecipitation, and bimolecular fluorescence complementation, the research team confirmed that VirE2 can directly interact with the cargo binding domains (CBDs) of myosin VIII-2, VIII-A, VIII-B, and myosin XI-K, but the direct interaction with the CBD of myosin VIII-1 was very weak or undetectable. Subcellular localization analysis showed that VirE2 significantly co-localized with myosin VIII-2, VIII-A, and VIII-B at the cell periphery (plasma membrane region). When myosin XI-K was knocked down in Nicotiana benthamiana using RNAi technology, or when its dominant-negative CBD was expressed in Nicotiana benthamiana/Arabidopsis, although the movement of VirE2 along actin filaments in the cytoplasm was completely blocked, this did not affect the efficiency of Agrobacterium-mediated transformation. Therefore, the intracellular motility of VirE2 may not be its sole or essential function in promoting transformation.
The researchers observed that VirE2-Venus protein expressed in plant cells was mainly localized to the cell periphery. This localization pattern of VirE2 remained unchanged when infected with Agrobacterium lacking T-DNA (virE2 mutant). However, when infected with Agrobacterium capable of transferring T-DNA, a portion of VirE2-Venus relocated from the cell periphery to the cytoplasm, with a significant increase in fluorescence signal, especially in the perinuclear region. This relocation was dependent on T-DNA transfer, not the presence of the bacteria itself.
When plants simultaneously expressed the CBDs of myosin VIII-A, VIII-B, or VIII-2, which sequester VirE2 at the membrane, T-DNA entry effectively released VirE2 from the cell periphery into the cytoplasm. However, expressing the CBDs of myosin VIII-1 or XI-K did not cause this significant redistribution of VirE2. This further supports the model that myosin VIII-2, VIII-A, and VIII-B sequester or anchor VirE2 at the plasma membrane region.
Real-time imaging analysis of VirE2 movement trajectories revealed that the movement speed of VirE2-Venus in the cell was unaffected in both wild-type and myosin VIII quadruple mutant backgrounds. However, when the CBD of myosin XI-K was expressed, VirE2 movement was completely inhibited. Similarly, VirE2 from Agrobacterium, after entering plant cells, moved along intracellular filamentous structures at a similar speed. Despite the inhibition of VirE2 movement, the transformation efficiency of Agrobacterium did not decrease under these conditions. Therefore, although myosin XI-K is the main driving force for the intracellular transport of VirE2, the rapid transport of VirE2 may not be absolutely necessary for successful T-DNA transformation.
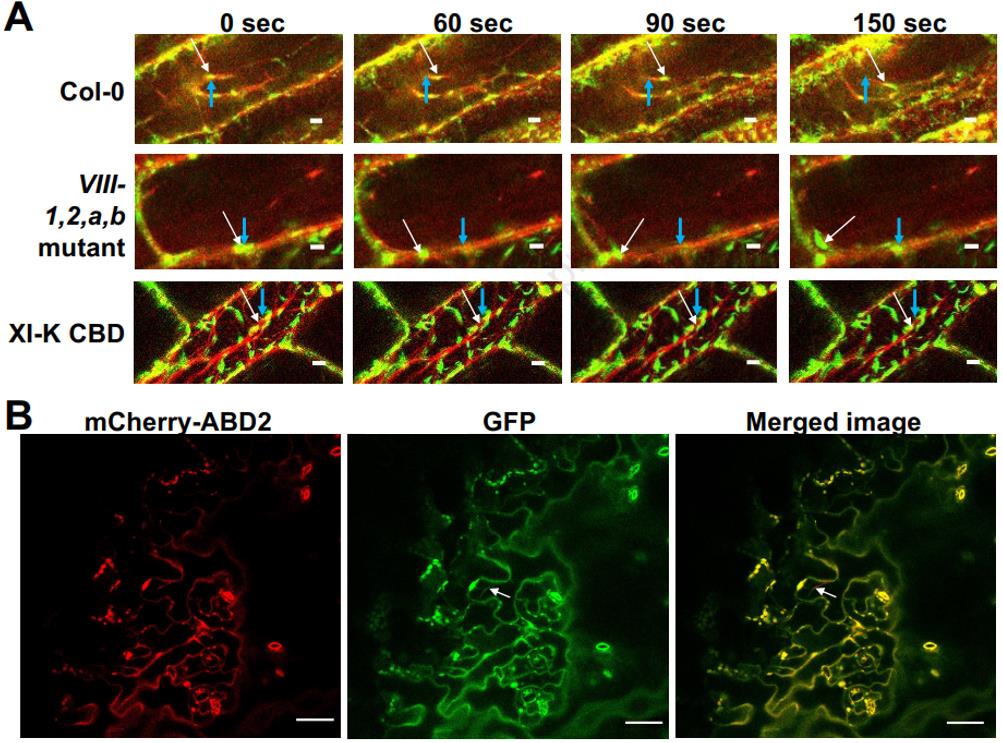
Figure 1. Movement of VirE2 along actin filaments. (Liu, et al. 2025)
In summary, this study reveals the synergistic division of labor mechanism of plant myosin VIII and XI in Agrobacterium-mediated transformation. Myosin VIII-2, VIII-A, and VIII-B act as anchoring proteins, responsible for tethering VirE2 to the plasma membrane. After the T-DNA/VirD2 complex enters the cell, VirE2 is activated and released from the plasma membrane; subsequently, myosin XI-K is responsible for driving VirE2 (possibly along with T-DNA) along the actin-endoplasmic reticulum system towards the perinuclear region.