On November 10, 2025, a research team led by Professor Nam-Soo Jwa from Sejong University published a research paper titled "Rice phospholipase C4 enhances Ca2+ and ROS bursts during effector-triggered immunity leading to ferroptotic cell death" in Plant Communications. This study revealed the crucial role of rice phospholipase C4 (OsPLC4) in mediating ferroptosis in rice, providing a new perspective for understanding the molecular mechanisms of ferroptosis in plants.
Ferroptosis is a type of programmed cell death regulated by iron ions and reactive oxygen species (ROS), playing a key role in plant immune responses. When rice is infected with avirulent rice blast strains, intracellular Ca2+ influx and ROS bursts synergistically trigger ferroptosis, thereby activating defense responses.
Phospholipase C (PLC) is an important enzyme connecting lipid signaling and Ca2+ regulation, but its mechanism of action in rice immunity is still unclear. This study found that rice phospholipase C4 (OsPLC4) promotes ferroptosis during effector-triggered immunity (ETI) by regulating Ca2+ and ROS levels, providing a new molecular mechanism for understanding the plant disease resistance signaling network.
Imaging of rice leaf sheath cells using Fluo-5F AM showed no significant difference in intracellular Ca2+ levels between PO6-6 (virulent) and INA168 (avirulent) treatments at 0–6 dpi; however, at 36–48 hpi, the green fluorescence in INA168-treated rice cells was significantly enhanced, accompanied by hypersensitive response (HR), while this phenomenon was not observed in PO6-6-treated rice cells, and extracellular (apoplast) Ca2+ significantly increased at 36 hpi. qRT-PCR results showed that OsPLC4 expression was significantly increased at 6–24 hpi after INA168 treatment, while it remained at a low level after PO6-6 treatment; simultaneously, the IP₃ content continuously increased at 6–48 hpi after INA168 treatment and was significantly higher than that after PO6-6 treatment, which is consistent with the OsPLC4-induced and continuously increasing Ca2+ content. In terms of disease resistance phenotype, ∆Osplc4 plants showed increased fungal hyphal growth in the leaf sheath, reduced cellular HR, increased leaf lesion area, and increased fungal biomass. At the molecular level, OsRbohB, OsMEK2, OsMPK1, and OsPAL1 were rapidly induced in the wild type (WT), while the expression of these genes was significantly suppressed in ∆Osplc4 (1H). These results indicate that OsPLC4 is a key member of the rice PLC family that mediates sustained Ca2+ influx and defense activation during ETI.
Upon infection with a non-toxic strain of M. oryzae, wild-type rice exhibited significant intracellular Ca2+ influx, ROS and Fe3+ accumulation, lipid peroxidation, and HR cell death. These responses were significantly suppressed in the ΔOsplc4 mutant. The mutant showed decreased IP3 levels, absence of ROS and Fe3+ signals, reduced MDA levels, and no HR, indicating that OsPLC4 mediates ferroptotic cell death by regulating the IP3-dependent Ca2+ signaling pathway.
Treatment with different chemical substances revealed that Ca2+ signaling is crucial in OsPLC4-mediated ferroptosis. The Ca2+ chelator EGTA significantly inhibited the accumulation of Ca2+, ROS, and Fe3+, as well as HR cell death; neomycin (a PLC inhibitor) and nifedipine (a Ca2+ channel blocker) partially reduced these responses. Conversely, TFP (a calmodulin binding agent) and ASM (a salicylic acid analog) significantly enhanced Ca2+ influx, ROS and Fe3+ accumulation, and HR cell death. These results indicate that Ca²⁺ influx inhibitors can suppress ferroptosis, while Ca2+ activators can promote ferroptosis.
The study proposes a mechanistic model of OsPLC4-regulated Ca2+-dependent ferroptosis. In effector-triggered immunity (ETI), extracellular Ca2+ is released and enters the cytoplasm through resistance bodies and Ca2+ channels. OsPLC4, located on the plasma membrane, is activated and hydrolyzes PIP2 to produce IP3 and DAG; IP3 promotes the release of Ca2+ from the endoplasmic reticulum, while the PA generated from DAG activates the MAPK and Rboh pathways, inducing ROS production. The interaction of Ca2+, ROS, and Fe2+ triggers lipid peroxidation and ferroptosis. EGTA, Neomycin, and NDP inhibit this pathway, while TFP and ASM enhance the response. This model reveals the crucial role of OsPLC4 in connecting Ca2+, ROS, and iron signaling and regulating plant immunity.
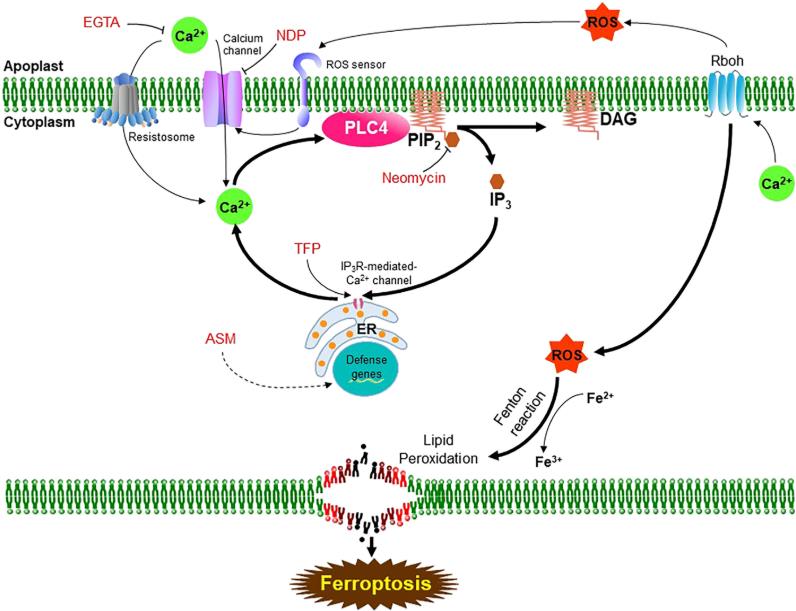
Figure 1. Proposed model of the phospholipase C4 pathway and its central role in the tight crosstalk between Ca2+ and ROS in Ca2+-mediated ferroptotic cell death downstream of resistosome activation. (Nguyen, et al. 2025)
This research represents the collective effort of Professor Nam-Soo Jwa's team at Sejong University. The team first discovered ferroptosis in rice during infection by the M. oryzae in 2019 and has since been dedicated to unraveling its molecular mechanisms, deeply exploring the synergistic mechanisms of calcium ions, reactive oxygen species, and iron signaling in regulating plant ferroptosis and immune responses.