Plant genetic transformation and gene editing technologies are the core driving force of modern agricultural breeding, enabling scientists to precisely improve crop varieties and endow them with superior traits such as high yield, disease resistance, and stress tolerance. However, this technology has long relied heavily on a cumbersome, lengthy, and costly process—tissue culture. Traditional procedures require placing a small piece of plant tissue (explant) on a culture medium containing artificial hormones, undergoing complex induction for months or even longer to regenerate a complete transgenic or gene-edited plant. Even more challenging is that the success rate of tissue culture is highly dependent on the plant species and genotype. Many important economic crops (such as soybeans and cotton) or superior cultivars are "resilient" and extremely difficult to regenerate in vitro, constituting a major bottleneck in the field of plant biotechnology that urgently needs to be overcome. Therefore, developing in vitro regeneration technologies that rely less or no on tissue culture, achieving direct in planta induced regeneration, has become a revolutionary goal pursued in this field.
On December 1, 2025, the team of Gunvant B. Patil from Texas Tech University published a paper in Molecular Plant titled "A synthetic transcription cascade enables direct in planta shoot regeneration for transgenesis and gene editing in multiple plants," reporting an ingenious and efficient integrated system for in planta regeneration, transformation, and gene editing in plants. This research successfully achieved the direct induction of heritable transgenic or gene-edited shoots from non-meristematic tissues in multiple plant species, including tobacco, tomato, and soybean, by designing a sophisticated "synthetic transcription cascade" switch that couples the plant's natural wound healing response with powerful organogenesis signals. This breakthrough technology provides a powerful solution to overcome the bottlenecks of traditional tissue culture and is expected to greatly accelerate the process of crop genetic improvement in the future.
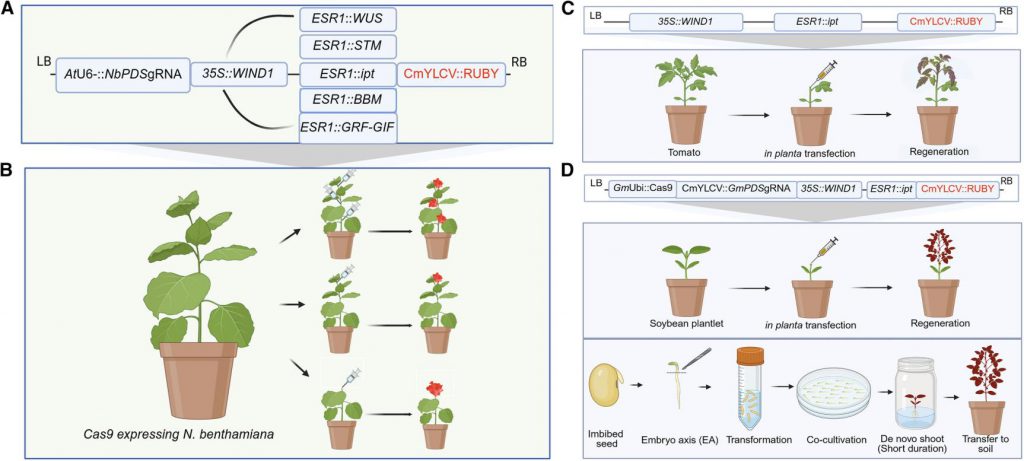
Figure 1. Schematic diagrams of the constructs and transfection strategies. (Kshetry, et al. 2025)
Plants possess powerful regenerative potential, especially after injury, where wound signals activate a series of genes to repair tissues. The research team cleverly utilized this natural mechanism. They designed a two-step gene regulatory circuit: first, using a promoter highly sensitive to wound signals, WOUND INDUCED DEDIFFERENTIATION 1 (WIND1); then, using it to initiate the expression of another key gene—the ENHANCER OF SHOOT REGENERATION 1 (ESR1) promoter. ESR1 itself is a "master switch" that promotes cell dedifferentiation and shoot regeneration. This "WIND1-driven ESR1" cascade design is equivalent to creating an intelligent response system that regenerates wherever there is injury. To verify the feasibility of this design, researchers introduced the WIND1 gene along with a red fluorescent reporter gene RUBY driven by the ESR1 promoter into tobacco. The experimental results clearly showed that the activity of the ESR1 promoter alone was weak, but in the presence of the WIND1 gene, significant red patches of callus tissue formed at the wound site, proving that WIND1 can indeed be activated by wound signals and effectively activate the downstream ESR1 promoter.
Activating ESR1 alone is not sufficient to induce complete organ regeneration. Therefore, the research team integrated several known key developmental regulatory factors (DRs) downstream of this cascade system, such as WUS, STM, BBM, and a key cytokinin synthesis gene ipt. They placed these genes under the control of the ESR1 promoter, constructing a series of WIND1-ESR1 (WE) vectors. After testing these combinations, a combination called WIND1 driving ESR1 activating ipt (WEipt) showed astonishing results. When Agrobacterium containing WEipt was injected into the internodes of young tobacco plants (a region completely devoid of meristematic tissue), a large number of new shoots grew at the injection site within only 12 days. This result indicates that WIND1 activated by the wound signal successfully initiated local, high-level expression of ipt, and the resulting cytokinin subsequently induced the formation of new meristems and bud regeneration. By optimizing the regeneration strategy (injection into the stem tip after pruning), the regeneration efficiency of this method reached 71% in tobacco, with approximately 35% of the new buds confirmed to be stably heritable transgenic buds.
The power of this platform lies not only in regeneration but also in its seamless integration with gene editing technology. The research team integrated the WEipt system with CRISPR-Cas9 components into a single vector, with the gRNA targeting phytoene desaturase (PDS), a gene responsible for chlorophyll synthesis in plants. Knocking out this gene results in albino plants, providing a visual indicator of successful gene editing. When this integrated "regeneration and editing" vector was injected into tobacco plants, researchers observed three types of regenerated shoots at the wound site: non-transgenic green shoots, red shoots containing only the reporter gene, and white (albino) shoots where gene editing had successfully occurred. Gene sequencing confirmed that the PDS gene in these albino shoots had indeed undergone the expected DNA sequence deletion, achieving gene knockout. This result dramatically demonstrates that the system can simultaneously induce regeneration and complete gene editing in a single step, directly obtaining asexually propagated gene-edited plants, greatly simplifying the process.
To verify the universality of this technology, the research team applied it to tomatoes, which are closely related to tobacco, and soybeans, which are distantly related and notoriously difficult to transform. In tomatoes, using a strategy similar to that used in tobacco, the WEipt vector was injected into the stems of pruned seedlings, successfully inducing a large number of transgenic shoots carrying the red marker within 3 weeks, with a transformation efficiency of 21%. Although the fertility of these first-generation transgenic tomatoes was somewhat affected, this experiment successfully demonstrated the versatility of this technology in Solanaceae crops.
For soybeans, a "recalcitrant" crop, direct injection into the stem did not successfully induce shoot regeneration. Therefore, the research team developed an innovative "semi-in vitro" method. They excised the hypocotyls of soybean seeds as explants, performed Agrobacterium-mediated transformation and brief co-cultivation under sterile conditions, and then transferred them to a culture medium without any hormones. Thanks to the powerful regeneration capabilities of the WEipt system, these hypocotyl explants developed a large number of transgenic and gene-edited shoots within 28 days. The regeneration efficiency of this method reached 80%, the transgenic efficiency was 28%, and the gene editing efficiency exceeded 12%, far surpassing traditional soybean tissue culture methods. This success not only overcame the challenges of soybean transformation but also provided a highly promising solution for other crops that are difficult to regenerate.
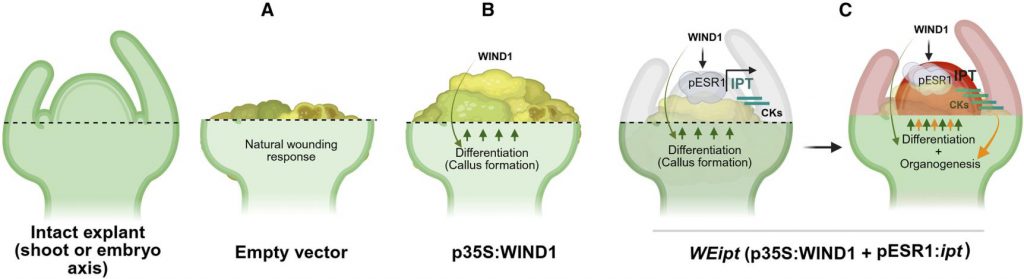
Figure 2. Schematic model illustrating de novo shoot regeneration. (Kshetry, et al. 2025)
This study, through the ingenious design of synthetic biology, created a programmable transcription cascade system (WEipt) initiated by wound signals, successfully "hijacking" and directing the plant's endogenous regeneration program towards our desired outcome. This system, through the synergistic action of "WIND1-mediated cell reprogramming" and "ipt-mediated organogenesis," achieved efficient and rapid de novo shoot regeneration in non-meristematic tissues of plants. The core breakthrough of this work is that it provides a universal, efficient, and user-friendly platform that eliminates or greatly simplifies the reliance on traditional tissue culture, thereby enabling rapid genetic transformation and gene editing in multiple plant species. It shortens the tissue culture cycle from months to weeks and has been successfully applied to tobacco, tomato, and, notably, overcome the regeneration difficulties in soybean. This "plug-and-play" regeneration module provides a revolutionary tool for accelerating functional genomics research and speeding up crop breeding processes, heralding a new era of more efficient and universal plant biotechnology.