Professional, Customizable Transformation Systems for Woody Plants
Lifeasible specializes in providing high-efficiency Liriodendron chinense (Chinese Tulip Tree) transformation services. As a valuable timber species and a critical model for studying basal angiosperm evolution, the Chinese Tulip Tree presents unique challenges in genetic engineering. Our platform utilizes an optimized somatic embryogenesis system, offering seamless support from vector design to the regeneration of transgenic plantlets. We provide tailored solutions for researchers focusing on wood formation, floral evolution, and abiotic stress resistance.
Stable transformation is the gold standard for analyzing gene function in woody plants. Our service is based on a highly efficient Agrobacterium-mediated transformation of embryogenic callus. This system has been rigorously optimized to overcome the recalcitrance often found in woody species.
Unlike traditional organogenesis methods, our somatic embryogenesis platform ensures high regeneration rates and minimizes the production of chimeric plants. This allows for the reliable generation of transgenic plantlets suited for long-term greenhouse or field studies.
![]()
Embryogenic Callus Induction
Induction of high-quality proembryogenic masses (PEMs) from immature zygotic embryos or shoot tips.
![]()
Agrobacterium Infection
Co-cultivation of activated PEMs with Agrobacterium tumefaciens carrying the target binary vector.
![]()
Selection of Transformants
Screening for resistant callus aggregates on selective media containing antibiotics (e.g., Kanamycin or Hygromycin).
![]()
Somatic Embryo Maturation
Inducing the differentiation of resistant callus into mature, cotyledonary somatic embryos.
![]()
Regeneration & Hardening
Germination of embryos into rooted plantlets, followed by acclimatization for greenhouse growth.
For researchers who need rapid validation of gene function or promoter activity without the months-long wait required for stable transgenic trees, our transient transformation services offer a powerful and efficient alternative. These systems are ideal for exploratory studies, subcellular localization, and protein-protein interaction analyses in Liriodendron chinense.
We offer a comprehensive suite of transient expression protocols to meet diverse research needs, ensuring successful delivery into woody plant tissues.
![]()
Construct & Material Preparation
Verification of plasmid vectors and preparation of healthy recipient tissues (leaves, callus, or protoplasts).
![]()
Delivery / Transfection
Introduction of DNA/RNA into cells via Agro-infiltration, PEG-mediated transfection, or Particle Bombardment.
![]()
Incubation
Short-term culture of transformed tissues (typically 24–72 hours) under controlled conditions to enable expression.
![]()
Signal Detection / Sampling
Observation of reporter signals (e.g., GFP/LUC) or tissue sampling for molecular analysis.
![]()
Data Analysis
Comprehensive analysis including confocal microscopy imaging, qRT-PCR, or enzyme activity reports.
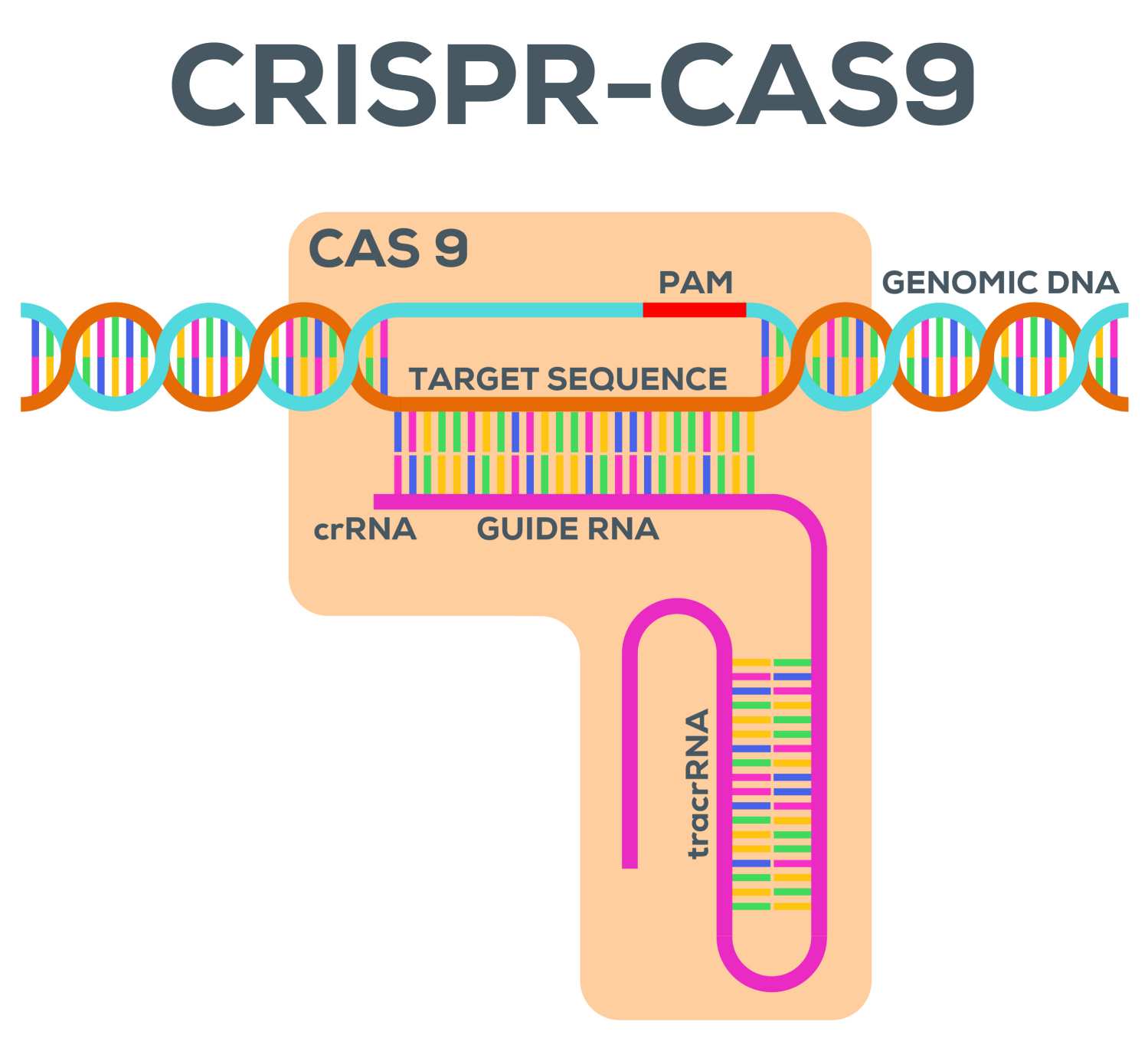
Our CRISPR/Cas9 platform for Liriodendron chinense enables precise genome engineering. Given the long generation time of woody plants, efficient editing in the T0 generation is critical. We utilize high-efficiency promoters to drive Cas9 expression specifically in embryogenic tissues.
To dissect gene regulatory networks, we offer custom construction of overexpression and RNA interference (RNAi) vectors tailored for woody plants.
Our team provides end-to-end molecular biology support, ensuring your vectors are optimized for expression in Liriodendron chinense.
Service Capabilities
Standard deliverables include
Optional Upgrades

Lifeasible employs a diverse and optimized toolkit to overcome the challenges associated with woody plant genetic engineering. We offer a selection of transformation methodologies to ensure successful DNA delivery into Liriodendron chinense tissues, catering to both stable integration and transient analysis requirements.
This is our primary method for generating stable transgenic lines and performing transient leaf assays. We utilize optimized Agrobacterium tumefaciens strains (e.g., EHA105, GV3101) and virulence-enhancing compounds (acetosyringone) to infect Liriodendron embryogenic callus or leaf tissues.
We utilize plant viral vectors to facilitate rapid gene function analysis. This method is particularly powerful for Virus-Induced Gene Silencing (VIGS), allowing researchers to quickly assess loss-of-function phenotypes in Liriodendron seedlings without the long wait for stable mutants.
PEG-mediated transformation is a chemical method used to induce direct DNA uptake by cells. This technique is highly effective for transforming isolated protoplasts or specific cell types where Agrobacterium infection may be less efficient.
For tissues that are recalcitrant to biological vectors, we employ biolistic delivery. This physical method uses high-velocity gold or tungsten particles coated with DNA to penetrate the cell wall and deliver genetic material directly into the nucleus.
Vector Construction
Embryogenic Callus Induction & Preparation
Agrobacterium Infection & Co-cultivation
Somatic Embryo Induction & Maturation
Plantlet Regeneration & Rooting
Molecular Verification (PCR)
Total Estimated Time: 8-12 months depending on construct and genotype.
Species-Specific Expertise
Our team has deep experience in woody plant tissue culture, specifically overcoming the "recalcitrant" nature of Liriodendron.
Optimized Protocols
We utilize a proprietary somatic embryogenesis system that significantly outperforms traditional organogenesis methods.
Customized Solutions
Tailored strategies for functional genomics in basal angiosperms.
End-to-End Support
From vector design to greenhouse-ready saplings.
Ready to advance your research on basal angiosperms?
Contact us for a free consultation regarding your Liriodendron chinense transformation project.
Liriodendron chinense is a genus of Magnoliaceae. Distributed in China and northern Vietnam, it has peculiar leaf shape and colorful flowers and is an excellent ornamental tree species for garden cultivation and landscaping. At the same time, its tree body is tall; the wood structure is detailed and uniform, it is an excellent pulp and paper, man-made boards and furniture timber species, and the social demand is excellent. But hybrid Liriodendron chinense growth cycle is long, and the application of conventional means of breeding has more significant difficulties. Therefore, it is necessary to rely on modern biotechnology and combine it with conventional breeding to shorten the breeding cycle, accelerate the breeding process, create high-quality plantation forests, and alleviate the contradiction between timber supply and demand.
Unlike the floral dip method used in Arabidopsis, Liriodendron transformation relies on a sophisticated Somatic Embryogenesis (SE) system. This process involves inducing proembryogenic masses (PEMs) from immature zygotic embryos. Agrobacterium-mediated transformation of these PEMs allows for the generation of transgenic somatic embryos, which then germinate into plantlets. This method is superior to organogenesis for woody plants as it minimizes chimerism and ensures that the transgene is integrated into the germline for stable inheritance.
Liriodendron chinense (Chinese Tulip Tree), a member of the Magnoliaceae family, holds a unique position in the plant kingdom as a basal angiosperm. It serves as a critical reference point for understanding the evolution of flowering plants, distinct from monocots (e.g., rice) and eudicots (e.g., Arabidopsis). Transforming Liriodendron allows researchers to perform comparative genomics and Evo-Devo studies, shedding light on the ancestral functions of genes regulating flower development and vascular evolution.
As a fast-growing timber species, the Chinese Tulip Tree is an excellent model for studying secondary cell wall biosynthesis and wood formation. Transformation services facilitate the functional characterization of genes involved in lignin and cellulose pathways. By manipulating these genes, researchers can develop varieties with improved wood density, altered fiber composition, or reduced recalcitrance for biofuel production.
Liriodendron species are rich in bioactive secondary metabolites, including terpenoids and alkaloids (e.g., aporphine alkaloids), which have significant pharmaceutical value. Through metabolic engineering, transgenic technology can be used to overexpress key biosynthetic enzymes or silence competitive pathways. This enables the identification of gene clusters responsible for these compounds and the development of cell lines with enhanced production of high-value phytochemicals.
Transformation of woody plants is historically "recalcitrant" due to long regeneration cycles, low efficiency, and genotype dependence. Common hurdles include tissue browning (oxidation) and difficulty in rooting. Our platform addresses these challenges by using optimized anti-browning agents, specific Agrobacterium strains (such as EHA105 or GV3101), and tailored hormone ratios, significantly shortening the timeline from callus to plantlet compared to traditional protocols.
You can provide the plasmid vector or the gene sequence. Since Liriodendron transformation is complex, we recommend using our validated backbone vectors.
Unlike Arabidopsis, woody plants require a longer regeneration phase. The typical timeline is 9-12 months from vector construction to rooted plantlets.
Yes. We use Agrobacterium-mediated integration into the nuclear genome, ensuring the transgene is stable and heritable (though generation time is long).
Yes, our protocol is adaptable to L. chinense, L. tulipifera, and their hybrids.
Yes, we have successfully applied CRISPR/Cas9 in Liriodendron for gene knockout studies.