Plant transformation technology involves the introduction of foreign genes into the plant genome, enabling targeted modification of plant genetic traits, thereby promoting agricultural modernization and biotechnology development. This technology not only provides new tools for crop improvement but also demonstrates broad potential for applications in areas such as nutritional enhancement, stress tolerance enhancement, and pharmaceutical compound production. With the advancement of gene-editing technologies such as CRISPR-Cas9, plant transformation has evolved from traditional random integration to precise regulation, becoming a key tool for addressing global food security and environmental challenges.
Plant transformation technology is a core means of genetic improvement by introducing exogenous genes into the plant genome. Various methods exist, primarily including the following:
Agrobacterium tumefaciens mediates the stable integration of exogenous genes via the T-DNA region of the Ti plasmid. By modifying the T-DNA of the Ti plasmid, the target gene can be integrated into the plant genome, allowing plants to be regenerated through tissue culture. Agrobacterium-mediated transformation is simple and efficient, and has been widely used in dicotyledons (such as tobacco and tomato). However, monocotyledons (such as corn and wheat) lack actively dividing cells, resulting in lower transformation efficiency. Therefore, optimization of the explant type (such as immature embryos) and Agrobacterium strain (such as highly virulent strains) is necessary to increase transformation success rates.
Gene gun technique uses high-pressure gas to accelerate metal microparticles (such as gold powder) coated with the target gene through plant cell membranes, achieving gene delivery. This method is applicable to a wide range of plants, but suffers from low transformation efficiency, random integration of the exogenous DNA, and poor genetic stability.
Plant transformation technology imparts traits such as stress tolerance, improved nutrition, and herbicide resistance to crops by stably integrating foreign genes into the plant genome. Specific application areas include:
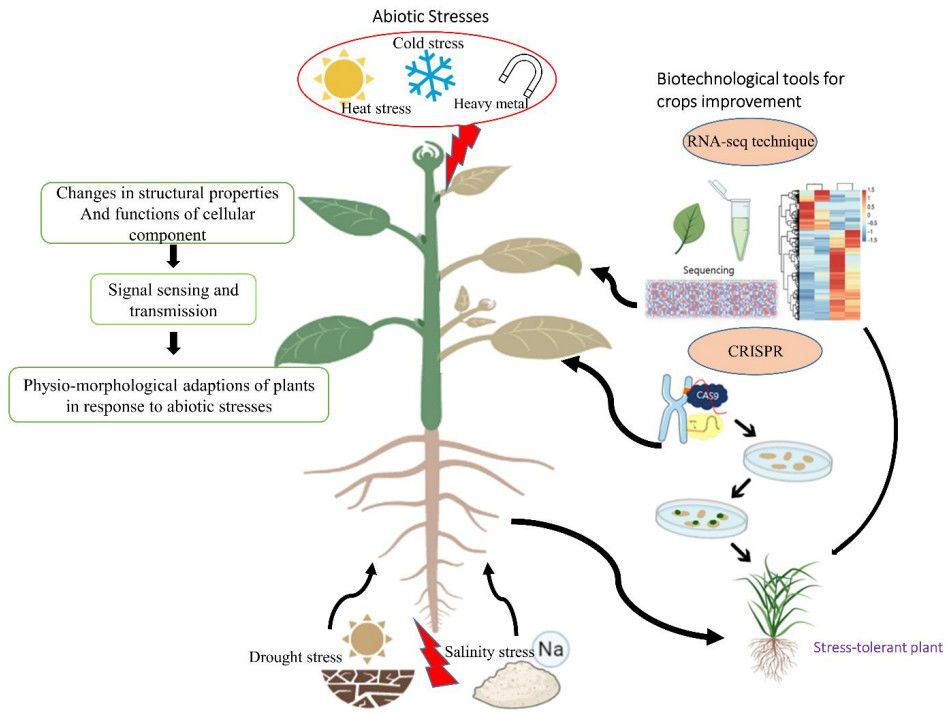 Fig. 1. Major abiotic stresses and biotechnological tools to help plants to resist these stress conditions. (Imran, et al. 2021)
Fig. 1. Major abiotic stresses and biotechnological tools to help plants to resist these stress conditions. (Imran, et al. 2021)
Through genetic engineering, scientists can precisely manipulate a plant's flowering time, plant architecture, and sink-source relationships, thereby optimizing light energy utilization efficiency and photosynthate distribution. For example, introducing genes that control plant architecture (such as dwarfing genes) can reduce ineffective tillering and increase yield per unit area. Furthermore, the introduction of growth regulators (such as gibberellins or cytokinins) can further promote robust plant growth. In practical applications, insect-resistant corn (such as Bt corn) significantly reduces pest damage by expressing Bacillus thuringiensis toxin genes, indirectly increasing yield. Gene editing technologies (such as CRISPR-Cas9) can also target yield-related quantitative trait loci (QTLs), directly introducing high-yield alleles and accelerating breeding efforts.
Gene-editing technologies such as CRISPR-Cas9 enable targeted improvement of crop traits by precisely targeting genomic loci. For example, CRISPR-Cas9 has been successfully used to improve disease resistance in rice (e.g., knocking out genes associated with rice blast) and enhance drought resistance in wheat. Compared to traditional transgenics, gene-editing technologies do not require the introduction of foreign DNA, reducing regulatory risks. Furthermore, multi-gene editing (e.g., simultaneous editing of multiple resistance genes) allows for the rapid development of multi-resistance varieties.
Synthetic biology approaches can be used to engineer crop secondary metabolic pathways to produce high-value-added compounds. For example, gene editing has been used to overexpress the β-carotene synthesis gene in rice, resulting in the development of vitamin A-rich golden rice. Furthermore, the design of novel biosynthetic pathways (e.g., engineering plants to produce antibiotics or industrial enzymes) can provide sustainable raw materials for the pharmaceutical and industrial sectors.
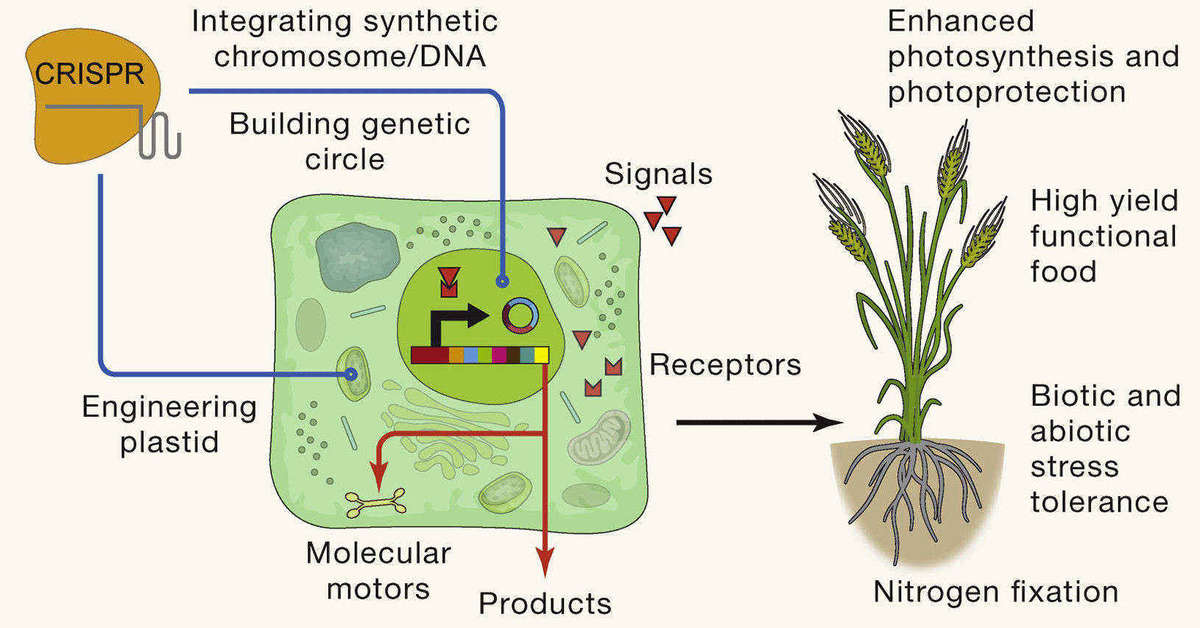 Fig. 2. CRISPR-mediated plant synthetic biology in which plant cell behavior is altered to enhance plant growth and product generation. (Gao, C. 2021)
Fig. 2. CRISPR-mediated plant synthetic biology in which plant cell behavior is altered to enhance plant growth and product generation. (Gao, C. 2021)
The global GM crop market is centered around soybean, corn, cotton, and canola. Data from 2018 showed that GM soybean cultivation accounted for 50% of the global GM crop area, followed by corn (30%), cotton (25%), and canola (20%). China, the United States, Brazil, India, and other countries are major producers. The United States has an average adoption rate of 93% for GM soybean, corn, and cotton, demonstrating the efficiency of technology adoption. Furthermore, the area of biotech crops with stacked traits (such as drought and insect resistance) has grown significantly, accounting for 42% of the global biotech crop area in 2018, demonstrating strong farmer demand for multifunctional traits.
The commercialization of biotech crops has significantly boosted agricultural productivity. Since their first commercialization in 1996, biotech crop yields have increased 87-fold, providing a stable food supply for 7.4 billion people worldwide. For example, herbicide resistance reduces pesticide use and lowers production costs, while insect resistance reduces pest losses and increases crop survival rates. Furthermore, biotech technology has promoted sustainable agricultural development. For example, salinity-alkaline tolerance has been added to the energy grass switchgrass, enabling its successful cultivation in saline-alkali lands and contributing to ecological restoration.
Despite significant progress in transgenic technology, its application still faces multiple challenges. First, the bottleneck of transformation efficiency is a core issue. Many crops (such as rice and wheat) are insensitive to traditional tissue culture and regeneration conditions, resulting in transformation rates as low as 10% or below. Second, public concerns arise about off-target effects and the risk of gene flow. For example, gene editing technologies like CRISPR-Cas9 may accidentally insert non-target genes or spread to wild populations through pollen. Furthermore, insufficient public acceptance limits the technology's widespread adoption, especially in areas with religious or cultural sensitivities.
Future technological development will focus on the following areas:
To achieve the sustainable development of transgenic technology, international cooperation and regulatory coordination are necessary. For example, establishing unified off-target effect detection standards and gene flow monitoring systems can enhance public trust. At the same time, developing countries need to narrow the technological gap with developed countries through policy support and capacity building.
As a core driver of modern agriculture, plant transformation technology has profoundly changed the paradigm of crop improvement. From early Agrobacterium-mediated transformation to modern gene editing, continuous technological advancements have provided innovative solutions to address food security, climate change, and resource scarcity. However, its development must balance technological innovation with social ethics. International cooperation and policy coordination should be used to promote the sustainable application of transgenic technology.
At Lifeasible, we specialize in providing cutting-edge plant transformation services to meet your agricultural and biotechnology research needs. Our expertise in genetic engineering and gene editing technologies enables us to provide precise and efficient transformation solutions for a wide range of plant species. Whether you're looking to enhance crop traits such as stress tolerance, nutritional value, or pest and disease resistance, we offer comprehensive services to meet your specific project needs.
Explore the Various Plant Species for Which We Offer Transformation Services
To help you select the right transformation service, we've provided a detailed table listing the plant species we support. This table will help you understand the scope of our capabilities.
Table1. Plant Species Covered by Lifeasible Transformation Services
| Category | Plant Name | ||
| Model Plant |
|
|
|
| Food Crops |
|
|
|
| Cash Crops |
|
|
|
| Vegetables |
|
|
|
| Fruits |
|
|
|
| Medicinal Plants |
|
|
|
| Ornamental Plants |
|
|
|
| Herbs |
|
|
|
| Woody Plants |
|
|
|
| Aquatic Plants |
|
|
|
| Fungi |
|
|
|
| Others |
|
|
|
Partner with Lifeasible for your plant transformation project and leverage our expertise to efficiently achieve your research goals. Contact us today to learn more about how we can support your research.
Q: What is plant transformation technology?
A: Plant transformation technology refers to the introduction of foreign genes into the plant genome, thereby modifying its genetic traits. It is used for crop improvement and biotechnology advancements.
Q: How does Agrobacterium-mediated transformation work?
A: This method uses Agrobacterium tumefaciens to integrate foreign genes into the plant genome via the T-DNA region of the Ti plasmid and is primarily applicable to dicotyledons.
Q: What are stable and transient transformation?
A: Stable transformation involves the integration of foreign genes into the plant genome for heritability, while transient transformation is temporary and suitable for short-term research.
Q: What are the applications of plant transformation in agriculture?
A: Applications include enhancing resistance to biotic and abiotic stresses, improving nutritional value, enhancing herbicide resistance, increasing crop yield, and extending shelf life.
Q: What challenges does plant transformation technology face?
A: Challenges include low transformation efficiency, potential off-target effects, the risk of gene flow, and public acceptance issues.
Q: What are the socioeconomic benefits of commercialized genetically modified crops?
A: Genetically modified crops have increased agricultural productivity, reduced pesticide use, lowered production costs, and promoted sustainable agricultural development.
References