Pioneering Platforms for Early Land Plant Research
Lifeasible offers a comprehensive suite of transformation services for bryophytes, empowering research in plant evolution, synthetic biology, and metabolic engineering. Recognizing that "one size does not fit all," we provide a selection of specialized transformation methods tailored to different bryophyte lineages. From the unique homologous recombination capability of Physcomitrium patens to the rapid Agrobacterium systems for Marchantia polymorpha, our platform provides end-to-end support from vector construction to mutant library generation.
For the model moss Physcomitrium patens, PEG-mediated protoplast transformation is the gold standard. This method leverages the moss's unique ability for high-efficiency homologous recombination (HR), allowing for precise gene targeting that is difficult to achieve in vascular plants.
Our optimized protocol ensures high protoplast yield and regeneration rates. This method is ideal for generating precise knock-ins, knockouts, and performing allele replacements directly in the haploid gametophyte.
![]()
Protoplast Isolation
Enzymatic removal of the cell walls from fresh protonemal tissue or callus to yield viable single-cell protoplasts.
![]()
DNA/Vector Preparation
Preparation of linearized DNA or plasmid vectors optimized for high-efficiency homologous recombination or random integration.
![]()
PEG-Mediated Transfection
Mixing protoplasts with DNA and Polyethylene Glycol (PEG) under specific conditions to facilitate direct DNA uptake into the cells.
![]()
Selection & Regeneration
Culturing the protoplasts in soft agar, followed by regeneration and selection on medium containing antibiotics or herbicides.
![]()
Genotype Verification
Genomic DNA extraction from regenerated tissue for verification of gene integration and targeting efficiency via PCR or sequencing.
For the liverwort Marchantia polymorpha, Agrobacterium-mediated transformation of spores or thallus fragments (gemmae) is the most efficient method. Unlike the floral dip in Arabidopsis, this method involves co-cultivation of vegetative tissues or spores with Agrobacterium, leading to rapid generation of stable transgenic lines.
![]()
Agrobacterium Preparation
Activation and culture of Agrobacterium strains carrying the target vector, followed by the preparation of a high-density cell suspension.
![]()
Spore/Gemmae Inoculation
Co-cultivation of Marchantia spores or shoot cups (gemmae) with the Agrobacterium suspension for T-DNA transfer.
![]()
Clearance & Washing
Removing excess Agrobacterium using antibiotics and repeated washing to prevent overgrowth and subsequent necrosis of liverwort tissues.
![]()
Selection & Differentiation
Culturing the tissue on selective media containing marker agents to isolate and induce the differentiation of resistant transformed thalli.
![]()
Stable Line Confirmation
Molecular verification (e.g., PCR) of independent regenerated thalli to confirm the stable and successful T-DNA integration into the genome.
For non-model bryophytes or tissues that are recalcitrant to biological vectors (like Agrobacterium) and chemical methods, our Particle Bombardment (Gene Gun) service offers a highly effective physical delivery alternative. This method bypasses the cell wall barrier entirely, allowing for DNA delivery into a broad range of tissue types, including mature spores, specialized tissues, and thick thalli, where other methods fail.
![]()
Microcarrier Preparation
Coating gold or tungsten microparticles with the target DNA/plasmid for precise delivery.
![]()
Target Tissue Selection
Selection and arrangement of the target bryophyte tissue (e.g., callus, thallus fragments, or spores) on the bombardment surface.
![]()
Bombardment
Firing the DNA-coated microparticles into the target tissue using controlled helium pressure.
![]()
Recovery & Selection
Culturing the bombarded tissue on regeneration media, followed by selection using appropriate antibiotics or herbicides.
![]()
Confirmation & Culture
Molecular screening (PCR) to confirm integration, followed by maintenance and proliferation of stable lines.
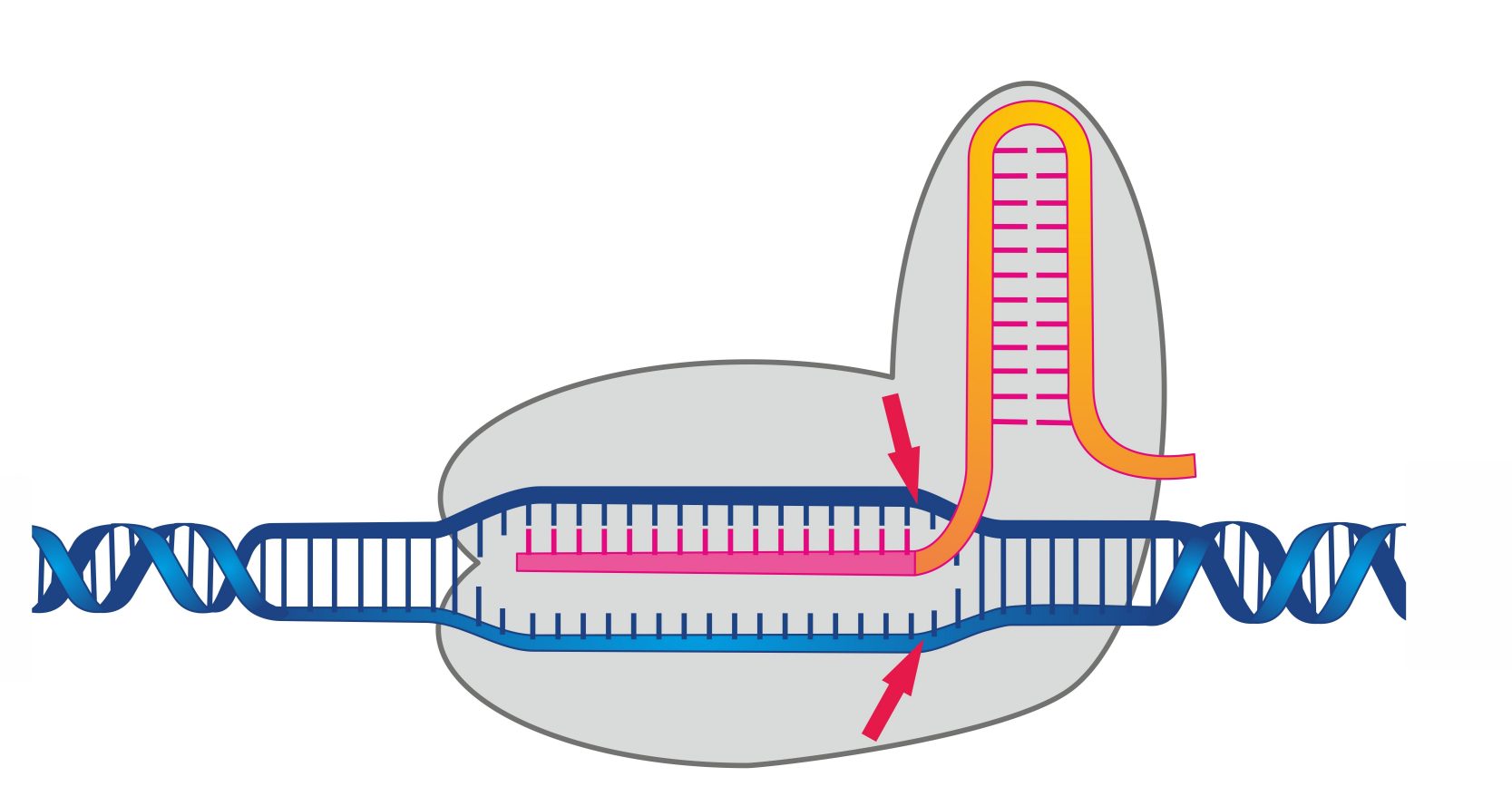
Bryophytes, with their dominant haploid phase, are excellent candidates for CRISPR/Cas9 editing, as phenotypes are directly visible without the need for complex segregation analysis.
Start Your Bryophyte Project Now
Bryophytes require specific regulatory elements different from angiosperms. We provide specialized vector construction services.

Standard deliverables include:
Optional upgrades:
Note: Bryophytes generally grow faster than woody plants but require specific care
Vector Construction
Tissue Preparation
Transformation (PEG or Agro)
Regeneration & Selection
Genotyping & Verification
Total: Approx. 3-4 months for stable lines.
Model & Non-Model Expertise
We handle standard Physcomitrium and Marchantia as well as emerging hornwort models.
Optimized Protocols
We employ tailored protocols for PEG, Agrobacterium, and Biolistics to ensure optimal success rates across different bryophyte species.
Custom Vectors
We possess a library of vectors specifically optimized for bryophyte expression levels.
End-to-End Support
From vector design to validated transgenic lines, we support your project every step of the way.
Leverage the unique genetic precision of bryophytes for rapid, targeted gene editing in model systems like P. patens and Marchantia.
Contact our experts today to select the optimal transformation method for your next functional genomics project.
Bryophytes (mosses, liverworts, and hornworts) represent the earliest diverging lineages of land plants. They are the "living fossils" of plant colonization. Transforming these species allows researchers to study the ancestral functions of genes found in crops today. For example, studying auxin signaling or stomatal development in bryophytes reveals how plants first adapted to dry land.
Physcomitrium patens (formerly Physcomitrella patens) possesses a superpower rare in the plant kingdom: efficient Homologous Recombination (HR). Unlike angiosperms that mostly integrate DNA randomly (NHEJ), P. patens can integrate DNA at specific genomic loci with high precision. This makes it the premier plant model for "reverse genetics" and precise gene replacement studies.
Bryophytes are emerging as powerful chassis for Synthetic Biology and Biopharming. They can be grown in sterile liquid cultures in bioreactors (photobioreactors), much like bacteria or yeast, but with the ability to perform complex post-translational modifications. This makes them ideal for producing complex pharmaceutical proteins, fragrances, or biofuels.
The dominant phase of the bryophyte life cycle is the haploid gametophyte. This is a massive advantage for genetic analysis. In diploid crops (like Rice or Arabidopsis), you must self-pollinate T1 plants and screen T2 progeny to find homozygous mutants. In bryophytes, the direct transformant is haploid—meaning if you knock out a gene, the phenotype is immediately visible. There are no "hidden" recessive alleles.
If you work with P. patens and need precise targeting, choose PEG-mediated. If you work with Marchantia or need simple overexpression, Agrobacterium-mediated is preferred.
It is one of the few multicellular organisms that exhibits high rates of homologous recombination in somatic cells, allowing for "yeast-like" gene targeting.
Bryophytes are dominant haploids. Therefore, a single transformation event usually results in a direct mutant phenotype without the need for selfing to create homozygotes (unlike Arabidopsis)