Plant transformation technology has profoundly reshaped the landscape of modern plant biology and agricultural biotechnology. From early explorations of genetic theory to today's sophisticated genetic engineering platforms, transformation technology has enabled researchers to insert, delete, or modify genes in plants with unprecedented precision. This article explores the history of transformation technology, its evolution over the decades, and recent advances in the field, highlighting the scientific milestones and technological innovations that have collectively defined its trajectory.
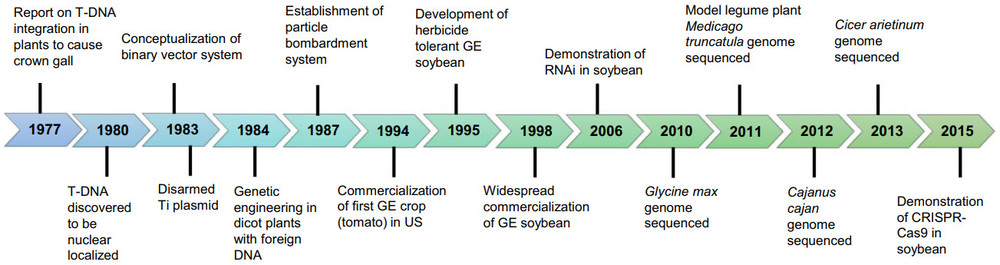 Fig. 1. Timeline of some of the major discoveries, applications and breakthroughs in the history of plant genetic transformation with special emphasis on legumes. (Choudhury, et al. 2021)
Fig. 1. Timeline of some of the major discoveries, applications and breakthroughs in the history of plant genetic transformation with special emphasis on legumes. (Choudhury, et al. 2021)
The early foundations of plant transformation technology were built on the understanding of the genetic nature of DNA, gene transfer mechanisms, and natural gene transfer systems. The discovery of Agrobacterium tumefaciens and the Ti plasmid laid a critical foundation for subsequent technological development, paving the way for the advent of plant genetic engineering.
The early foundations of plant transformation technology can be traced back to the mid-20th century, with its core concepts rooted in the exploration of the nature of genetic material and gene transfer mechanisms.
The early development of plant transformation technology is closely linked to the discovery of A. tumefaciens.
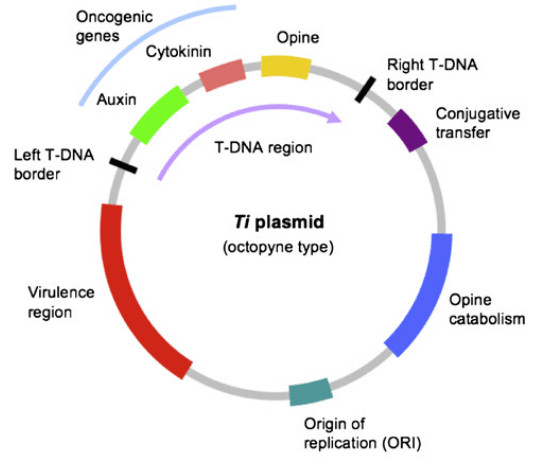 Fig.2. Ti plasmid. (Păcurar, et al. 2011)
Fig.2. Ti plasmid. (Păcurar, et al. 2011)
In the 1970s and 1980s, plant transformation technology centered on Agrobacterium-mediated transgenesis. Through the exploration of Agrobacterium-mediated T-DNA transfer and gene gun technology, the theoretical and technical foundations for transgenic plant research were established, laying the foundation for the subsequent diversification and modernization of technologies.
In the 1970s, researchers discovered that A. tumefaciens could transfer parts of its Ti plasmid into plant cells. This marked the first natural example of cross-kingdom gene transfer. By modifying the Ti plasmid, scientists could replace tumor-inducing genes with beneficial foreign genes, effectively utilizing Agrobacterium as a molecular vector.
In 1983, the first successful transgenic plants (e.g., tobacco) were created, marking a breakthrough in this technology. A. tumefaciens integrates foreign genes into the plant genome via the T-DNA contained in its Ti plasmid. This method involves modifying the Ti plasmid to delete the pathogenic gene and insert the target gene, then infecting the plant cells with Agrobacterium to achieve transformation. This process involves signal transduction, T-DNA replication, transfer, and integration, and its mechanisms were systematically elucidated in the 1980s.
These experiments demonstrated the feasibility of stable plant transformation and marked the true beginning of transgenic plant research.
Early Model Plants: Dicotyledonous plants such as tobacco and petunia served as model plants for early research due to their ease of transformation and regeneration.
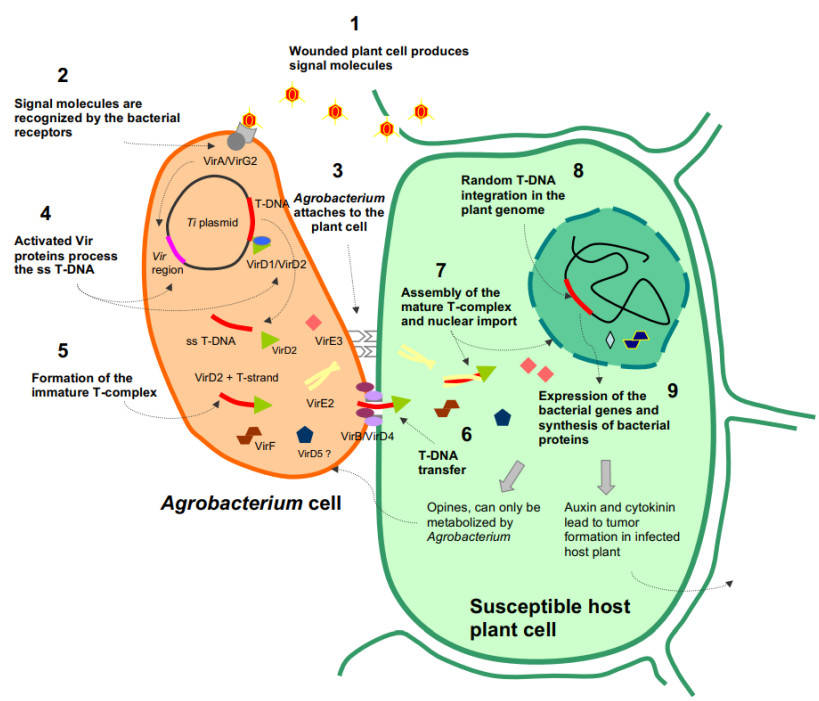 Fig.3. A simplified model of the Agrobacterium-mediated transformation process. (Păcurar, et al. 2011)
Fig.3. A simplified model of the Agrobacterium-mediated transformation process. (Păcurar, et al. 2011)
Gene Gun Technology: In the 1980s, the gene gun method (microprojectile bombardment) was developed. This method uses high pressure to inject DNA-encapsulated microparticles directly into plant cells for gene transfer. This method is not restricted to plant species, but suffers from low transformation efficiency and poor stability.
Agrobacterium-mediated transfection has become the mainstream method (accounting for over 80% of transgenic plant transformations) due to its high transformation efficiency, ability to integrate large DNA fragments, and strong stability. In contrast, the gene gun method, while simple to use, suffers from low transformation rates and issues such as exogenous gene silencing.
From the 1990s to the early 2000s, plant transformation technology achieved significant progress in vector systems, promoter design, marker genes, and selectable markers, driving the development of crop improvement and gene function research.
Early transgenics relied on random insertion, which often resulted in unstable expression or gene silencing. With the advent of molecular marker technology in the 1990s, scientists were able to better track and verify integration events.
After the 1990s, Agrobacterium-mediated transformation and electroporation became widely used for the transformation of cereals (such as corn and wheat) and monocots. For example, electroporation, which introduces DNA into protoplasts through electrical pulses, laid the foundation for the transformation of monocots. Furthermore, Agrobacterium-mediated transformation further promoted the application of genetic engineering in a variety of crops.
In plant genetic engineering, improvements in vector systems have become crucial. For example, the modification of Ti plasmids and the development of binary vector systems (such as Cis vectors and binary vectors) have improved gene transfer efficiency. In addition, the use of selectable markers and reporter genes in vector construction has further optimized transformation efficiency.
Promoter design has evolved from simple constitutive promoters to inducible and tissue-specific promoters, enabling more precise regulation of gene expression.
Marker genes (such as resistance genes) were initially used to screen transformed cells, but with technological advancements, the development of marker-free technologies has become a trend. Marker-free technologies improve biosafety by reducing the presence of foreign DNA. Reporter genes (such as the GUS reporter gene) are used to monitor gene expression.
The development of selectable marker systems (such as positive selection systems, phosphomannose isomerase) has addressed the biosafety concerns of traditional resistance markers.
Transgenic technology has become a complementary tool to traditional breeding. By the early 21st century, crops such as Bt cotton, herbicide-tolerant soybeans, and virus-resistant papaya demonstrated the powerful impact of transgenic technology on global agriculture.
This technique was developed in the late 1980s as an alternative to A. tumefaciens, particularly for monocotyledonous plants, which were initially difficult to transform. A gene gun is used to launch DNA-coated microparticles (gold or tungsten particles) into plant tissues. This method has expanded the host range and facilitated the transformation of rice, maize, and wheat.
Plant protoplasts are used to uptake DNA via electroporation or chemical methods. While theoretically powerful, challenges in regenerating fertile plants have limited their widespread application.
Nanoparticle-mediated gene delivery and viral vector technologies offer new avenues for gene delivery. These methods offer advantages in tissue culture freedom and genotype independence, making them suitable for gene editing in a wide range of crops. Plant viruses, such as tobacco mosaic virus (TMV), have been engineered to carry foreign sequences, enabling rapid but unstable expression and laying the foundation for transient transformation systems.
By engineering Agrobacterium strains and optimizing transformation methods (such as direct transformation of mature seeds or leaf segments), transformation efficiency has been improved and the operational process has been simplified. Furthermore, tissue culture-independent transformation methods reduce resource consumption and time costs.
Based on gene editing and functional genomics technologies, significant progress has been made in the breeding of stress-tolerant (such as drought and disease resistance) and high-yield crops. By targeting key genes (such as those associated with stress resistance), crop traits are improved, increasing yield and adaptability.
The CRISPR/Cas system represents a paradigm shift, providing precise and programmable gene editing. Unlike traditional transformation, CRISPR allows for targeted insertions, deletions, or base changes, typically without leaving foreign DNA.
Advances in synthetic promoters, gene circuits, and modular cloning systems have enabled more rational design of transgenic plants. These tools have expanded the scope of transformation from single-gene traits to engineering complex metabolic pathways.
Robotic systems for tissue culture and regeneration are accelerating throughput. Combined with genomic and phenomic data, these platforms make transformation more predictable and scalable.
Nanoparticles, carbon nanotubes, and peptide carriers are being investigated as alternative DNA delivery systems. These approaches could reduce reliance on tissue culture, which has long been a bottleneck for many plant species.
Currently, plant transformation technology faces multiple challenges. First, species dependence and genotype intractability are prominent, resulting in significant differences in transformation efficiency across different plants or genotypes, limiting the technology's universal applicability. For example, the complexity of multi-gene control, the random nature of exogenous gene integration, and the problem of multi-copy integration lead to insufficient transformation efficiency and stability. Furthermore, increasingly stringent regulatory and biosafety barriers, such as the complex approval process for gene-edited crops, and public concerns about biosafety also hinder technology adoption.
Future development focuses on technological innovation and application expansion. First, the development of a universal transformation platform is key, focusing on optimizing vector design, improving transformation efficiency, and leveraging AI and computational biology to enhance precision. Second, expansion to orphan crops and wild relatives will address food security and biodiversity concerns. Furthermore, ethical and social considerations must balance technological application with public acceptance to promote sustainable agricultural development.
The history of plant transformation technology reveals a remarkable scientific journey: from the discovery of the Agrobacterium Ti plasmid to today's cutting-edge genome editing and nanotechnology-driven platforms. Each decade, technological advancements have expanded the scope of plant biotechnology and enabled transformative applications in agriculture, bioenergy, and other fields.
As plant transformation technology continues to advance, its future depends not only on scientific breakthroughs but also on collaborative partnerships between researchers, industry, and service providers. These collaborative efforts will ensure that plant transformation remains at the core of innovation in plant science.
Lifeasible has developed a powerful platform for stable plant transformation and transient gene expression. Our team has expertise in Agrobacterium-mediated transformation, particle bombardment, and CRISPR-based genome editing, supporting researchers in crop improvement, metabolic engineering, and functional genomics.
Whether your project requires rapid transient assays for genetic validation or stable integration for trait development, we offer customized solutions to accelerate your research. We welcome collaborations from academic, industrial, and agricultural partners to advance plant transformation technology.
Q: What is plant transformation technology? Why is it so important?
A: Plant transformation technology involves the modification of plant genetic material, allowing for the insertion, deletion, or alteration of genes. It is important because it enables precise genetic improvement, improving crop yield, stress resistance, and adaptability.
Q: What role did A. tumefaciens play in the development of this technology?
A: A. tumefaciens and its Ti plasmid played a crucial role in enabling the first successful gene transfer from bacteria to plants. This natural gene transfer system laid the foundation for modern plant genetic engineering.
Q: How has gene gun technology contributed to plant transformation?
A: Developed in the 1980s, gene gun technology enables the direct delivery of DNA into plant cells, making it particularly useful for transforming monocots, thereby expanding the range of transformable plants, such as rice, maize, and wheat.
Q: How has CRISPR technology impacted plant transformation?
A: CRISPR technology has revolutionized plant transformation. It enables precise genetic modification without introducing foreign DNA, making it the preferred method for plant genome editing.
Q: What challenges does plant transformation technology face today?
A: These challenges include species-specific transformation efficiency, potential instability of transgene expression, regulatory hurdles, and public concerns about biosafety and genetically modified organisms.
Q: How do nanoparticles and viral vectors advance gene delivery in plants?
A: Nanoparticles and viral vectors offer new gene delivery methods that enable efficient and potentially tissue culture-independent transformation, thereby promoting rapid (albeit sometimes unstable) gene expression.
Q: What advances have been made in the design of plant transformation vector systems?
A: These advances include the development of binary vector systems that improve gene transfer efficiency and the use of marker-free and synthetic promoter systems for more precise and safer gene expression.
References