FDA Approves First Heterologous Skin Transplant Test: Skin Cells from Genetically Modified Pigs
Recently, the US Food and Drug Administration (FDA) has approved Xeno Therapeutics to begin the first clinical trial of xenogenic skin transplantation, which will use live skin cells from transgenic pigs to transplant into humans as a treatment for severe burns.
Xeno Therapeutics' bioactive Xeno-Skin product consists of the dermis and epidermal tissue layers from transgenic pigs that can be transplanted to humans after complete disinfection. The company said that previously, the viral pathogens of these animals have been an obstacle to the use of pig xenograft skin grafts. In addition, pigskin usually produces a sugar and human skin does not. In order to prevent pigs from producing this sugar, the company has genetically modified these pigs. As a result, pigskin is more likely to pass through the monitored of the transplanter's immune system. Therefore, the pigskin selected for this test is very similar to human skin, and these pig skins can be used for emergency burn treatment and human skin grafting.
“If this trial demonstrates the safety and efficacy of this new solution, it will be possible to address the clinical needs of emergency burn treatment options,” said Massachusetts General Hospital FACS Medical Doctor, Xeno-Skin clinical trial lead researcher Jeremy Goverman. “Xeno-Skin can be used to treat first-line treatments in the early stages of severe burns, and may also be used for prolonged temporary burn wound coverage.”
The skin is the largest organ in the body, preventing pathogens from invading the body's delicate internal organs, and thus plays a vital role in the immune system. In addition, the skin locks in moisture, electrolytes and other nutrients, and helps the body maintain body temperature. Severe burn patients are at risk due to the destruction of the skin barrier, the infection of opportunistic pathogens, the damage of immune response and the risk of fluid loss at the burn site, which then leads to the electrolyte, temperature, and acid-base imbalance. If not resolved, it will eventually cause organ failure and death. Therefore, during this critical period, burn patients need immediate treatment to restore normal body function as soon as possible in the acute phase of injury.
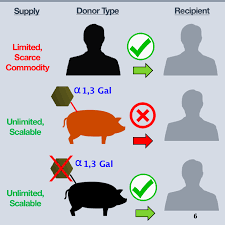
Currently, the only way to obtain transplanted skin in the United States is to use the skin left behind by the person who agrees to donate the organ or the skin of the patient who has undergone excess skin removal due to severe weight loss. Human skin for transplantation can be described as "scarce goods." “Xeno-Skin is the first non-human organ transplant approved by the FDA for research use,” said Paul Holzer, CEO, and co-founder of Xeno Therapeutics. “Our initial product goal is to advance the practicality of xenotransplantation therapy science, and to benefit the world's patients while addressing the clinical needs of patients with severe unmet burns; but equally important, the trial approval for this heterologous skin transplant has paved the way for future xenotransplantation solutions. The patient can also be protected during the healing process.
Xeno-Skin is a living cell xenograft product that contains a layer of epidermal and dermal cells that provides temporary wound coverage immediately during critical periods of burn treatment. It is intended as a replacement or supplement to the donor allograft, meaning that Xeno-Skin is intended to replace or complement existing donor allografts. Xeno Therapeutics said it will address this dilemma because Xeno-Skin is designed to be mass-produced, easily stored and transported when clinically needed.
Xeno Therapeutics is preparing to recruit eligible trial patients at the Massachusetts General Hospital by the end of the year. The non-profit company has been working closely with doctors at Massachusetts General Hospital, which will assist in clinical trials starting next month. The first clinical study will test the safety and tolerability of the transplant in only 6 severely burned patients. Assuming that the clinical trial results are positive after one month, the transplant work will be tested in two stages before being approved for clinical use.
Curtis Cetrulo, the chief medical officer of Xeno Therapeutics and a transplant surgeon at the Massachusetts General Hospital, said, “Ultimately, having a temporary skin barrier solution that can be stored frozen and shipped globally for immediate use or storage will help increase emergency preparedness for catastrophic events, and can greatly alleviate the current shortage of transplanted organs in the world.” Professor of Surgery, Columbia University Irvine Medical Center, Professor of Emeritus Surgery, Harvard Medical School, Laboratory of Transplantation Biology Research Center, Massachusetts General Hospital Dr. David H. Sachs said, "The use of genetically modified pigs for xenogeneic skin grafts may be a promising alternative to treating burns. This alternative source can avoid a range of risks of current use of dead donor allogeneic skin, including higher costs, limited donors, and human pathogen transmission."
For research or industrial raw materials, not for personal medical use!
 Currently, the only way to obtain transplanted skin in the United States is to use the skin left behind by the person who agrees to donate the organ or the skin of the patient who has undergone excess skin removal due to severe weight loss. Human skin for transplantation can be described as "scarce goods." “Xeno-Skin is the first non-human organ transplant approved by the FDA for research use,” said Paul Holzer, CEO, and co-founder of Xeno Therapeutics. “Our initial product goal is to advance the practicality of xenotransplantation therapy science, and to benefit the world's patients while addressing the clinical needs of patients with severe unmet burns; but equally important, the trial approval for this heterologous skin transplant has paved the way for future xenotransplantation solutions. The patient can also be protected during the healing process.
Xeno-Skin is a living cell xenograft product that contains a layer of epidermal and dermal cells that provides temporary wound coverage immediately during critical periods of burn treatment. It is intended as a replacement or supplement to the donor allograft, meaning that Xeno-Skin is intended to replace or complement existing donor allografts. Xeno Therapeutics said it will address this dilemma because Xeno-Skin is designed to be mass-produced, easily stored and transported when clinically needed.
Xeno Therapeutics is preparing to recruit eligible trial patients at the Massachusetts General Hospital by the end of the year. The non-profit company has been working closely with doctors at Massachusetts General Hospital, which will assist in clinical trials starting next month. The first clinical study will test the safety and tolerability of the transplant in only 6 severely burned patients. Assuming that the clinical trial results are positive after one month, the transplant work will be tested in two stages before being approved for clinical use.
Curtis Cetrulo, the chief medical officer of Xeno Therapeutics and a transplant surgeon at the Massachusetts General Hospital, said, “Ultimately, having a temporary skin barrier solution that can be stored frozen and shipped globally for immediate use or storage will help increase emergency preparedness for catastrophic events, and can greatly alleviate the current shortage of transplanted organs in the world.” Professor of Surgery, Columbia University Irvine Medical Center, Professor of Emeritus Surgery, Harvard Medical School, Laboratory of Transplantation Biology Research Center, Massachusetts General Hospital Dr. David H. Sachs said, "The use of genetically modified pigs for xenogeneic skin grafts may be a promising alternative to treating burns. This alternative source can avoid a range of risks of current use of dead donor allogeneic skin, including higher costs, limited donors, and human pathogen transmission."
Currently, the only way to obtain transplanted skin in the United States is to use the skin left behind by the person who agrees to donate the organ or the skin of the patient who has undergone excess skin removal due to severe weight loss. Human skin for transplantation can be described as "scarce goods." “Xeno-Skin is the first non-human organ transplant approved by the FDA for research use,” said Paul Holzer, CEO, and co-founder of Xeno Therapeutics. “Our initial product goal is to advance the practicality of xenotransplantation therapy science, and to benefit the world's patients while addressing the clinical needs of patients with severe unmet burns; but equally important, the trial approval for this heterologous skin transplant has paved the way for future xenotransplantation solutions. The patient can also be protected during the healing process.
Xeno-Skin is a living cell xenograft product that contains a layer of epidermal and dermal cells that provides temporary wound coverage immediately during critical periods of burn treatment. It is intended as a replacement or supplement to the donor allograft, meaning that Xeno-Skin is intended to replace or complement existing donor allografts. Xeno Therapeutics said it will address this dilemma because Xeno-Skin is designed to be mass-produced, easily stored and transported when clinically needed.
Xeno Therapeutics is preparing to recruit eligible trial patients at the Massachusetts General Hospital by the end of the year. The non-profit company has been working closely with doctors at Massachusetts General Hospital, which will assist in clinical trials starting next month. The first clinical study will test the safety and tolerability of the transplant in only 6 severely burned patients. Assuming that the clinical trial results are positive after one month, the transplant work will be tested in two stages before being approved for clinical use.
Curtis Cetrulo, the chief medical officer of Xeno Therapeutics and a transplant surgeon at the Massachusetts General Hospital, said, “Ultimately, having a temporary skin barrier solution that can be stored frozen and shipped globally for immediate use or storage will help increase emergency preparedness for catastrophic events, and can greatly alleviate the current shortage of transplanted organs in the world.” Professor of Surgery, Columbia University Irvine Medical Center, Professor of Emeritus Surgery, Harvard Medical School, Laboratory of Transplantation Biology Research Center, Massachusetts General Hospital Dr. David H. Sachs said, "The use of genetically modified pigs for xenogeneic skin grafts may be a promising alternative to treating burns. This alternative source can avoid a range of risks of current use of dead donor allogeneic skin, including higher costs, limited donors, and human pathogen transmission."