All organisms that reproduce sexually have two different sexes, and the developmental process involves sex determination. Therefore, sex determination and its molecular mechanisms are one of the most fundamental biological issues, and more importantly, the genetics and evolution of organisms cannot be separated from reproduction, and the continuation of species depends on the ability of embryos to form males or females.
Because of the special evolutionary position of fish among vertebrates, a large number of species, and the significant socioeconomic value, the study of sex determination in fish has always received attention from genetic and developmental scholars. A comprehensive understanding of the mechanism of sex determination in fish and its developmental biology can, on the one hand, study the evolutionary process of animals through sex differentiation, and on the other hand, sex dimorphism in cultured fish shows obvious differences in growth rates, which can be used to achieve unisexual culture through sex regulation and has important practical significance for aquaculture.
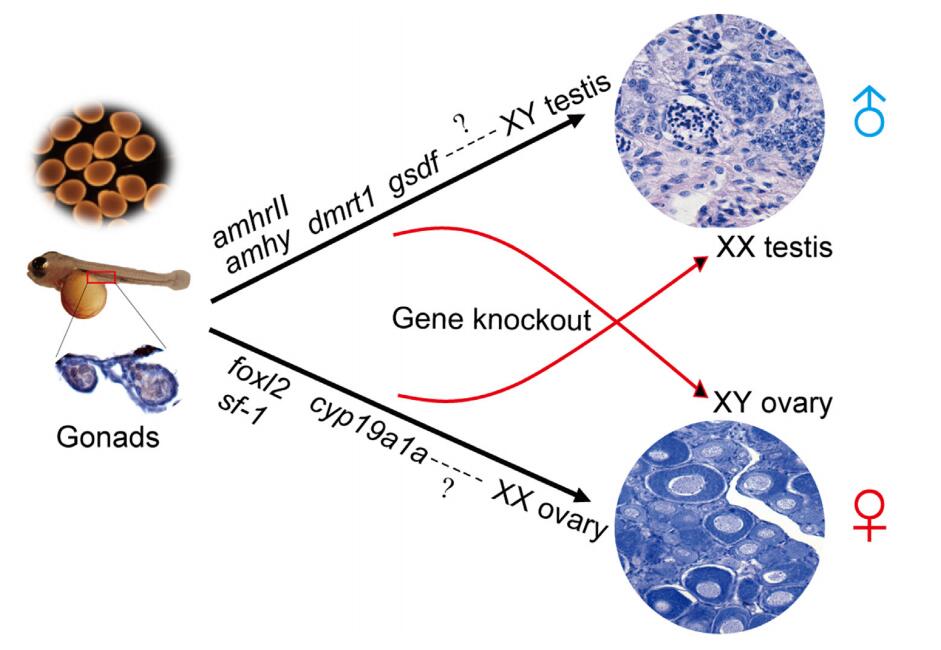 Figure 1. Genes identified in tilapia sex determination pathway. (Minghui L, et al. 2017)
Figure 1. Genes identified in tilapia sex determination pathway. (Minghui L, et al. 2017)
Tilapia has the incomparable advantages of other scleractinian fishes and is a good material for studying sex determination and differentiation, because its sexual maturation time is short, with an average spawning cycle of 14 days, and the number of eggs per tail can reach thousands, which makes it easy to obtain all-male and all-female fry. Tilapia males grow about 50% faster than females, and the culture of all-male fish has better economic value, because of its fast growth, strong adaptability, mixed food habits, fine and fresh meat, etc. It is recommended by the FAO to all countries in the world as an excellent breeding object.
 Figure 2. Tilapia swimming in the water.
Figure 2. Tilapia swimming in the water.
Gene-editing technology has been intensively studied not only in model fish such as zebrafish and medaka but also can be widely applied to economic fish such as tilapia. Lifeasible provides gene-editing technology for tilapia to prevent undesirable phenomena such as species degradation, reduced growth rate, reduced resistance, and reduced fertility. We use gene-editing technology not only to knock out genes and reveal gene functions and intrinsic mechanisms but also to edit target genes in an efficient and targeted manner, which significantly shortens the breeding time and is safer than traditional transgenic technology.
Key genes for gene editing
Genes associated with gonadal sex differentiation in tilapia, such as dmrt1, foxl2, igf3, nanos2, nanos3, Erβ, genes, etc.
CRISPR/Cas9 technology services
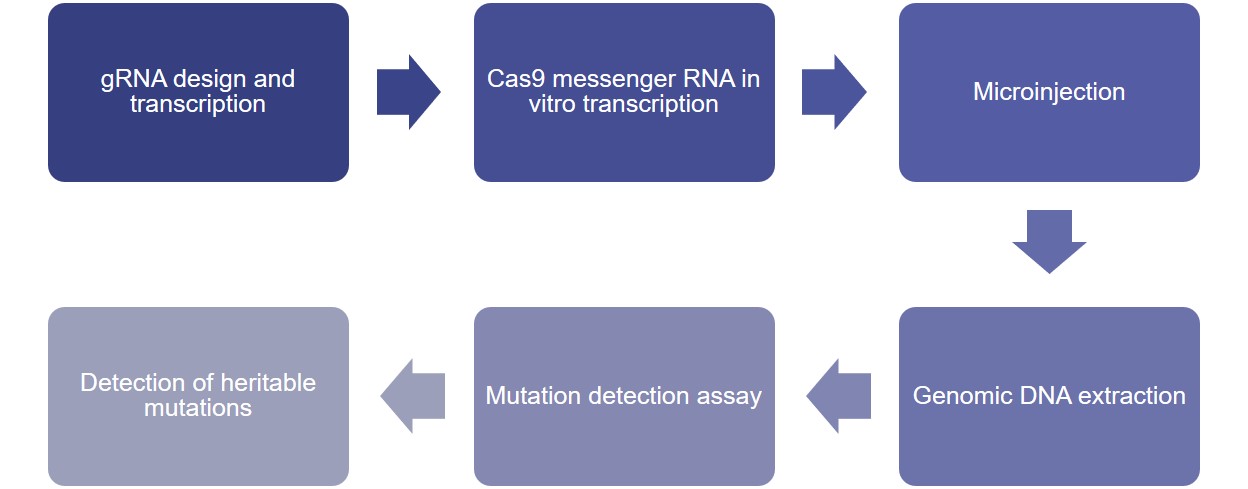
CRISPR/Cas9 has been proven to be effective for genome editing in several model organisms. Lifeasible can employ CRISPR/Cas9 for efficient and rapid knockdown of target genes in tilapia, such as Erβ, nanos, dmrt1, igf3, foxl2 genes, with a mutation rate of up to 95%. CRISPR/Cas9-induced gene mutations can produce significant phenotypes in G0 generation.
TALEN gene editing technology services
TALEN gene editing technology has been successfully applied to a variety of model organisms. Lifeasible has established TALEN-targeted gene knockout technology in tilapia to efficiently knock out target genes, such as dmrt1 and foxl2 genes (associated with gonadal sex differentiation), and our technology has achieved a maximum knockout rate of 81%.
In addition, individuals with a high mutation rate induced by TALEN can produce significant phenotypes in G0 generation, and we can detect the effect of target gene deletion on tilapia-related gene expression by conventional histology, immunohistochemistry, real-time PCR, and hormone assay at the request of our clients. For more information or any inquiry requirements, please contact Lifeasible.
References
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
STU-CRISPR System Improves Plant Genome Editing Efficiency
April 19, 2024
Application of Exosomes in Facial Beauty
April 12, 2024