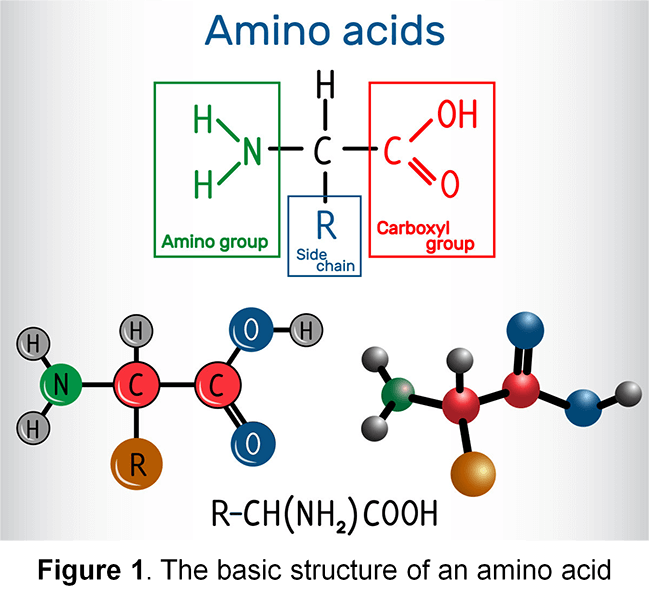
Amino acids are basic structural units of proteins. An amino acid typically consists of an amino group, a carboxyl group, a hydrogen atom, and a distinctive R group (Figure 1); all of which are bound to a carbon atom (except for Proline). There are 20 different naturally-existing types of amino acids. Amino acids can produce energy by being oxidized into carbon dioxide, water, and urea. Amino acids can also be used to synthesize carbohydrates, fats, hormones, and other substances. Accurate measurement of amino acid contents is essential for proper food production nutritional labeling, which is required for the assurance of food quality.
Lifeasible is your great partner for amino acid testing in food products. With decades of experience and highly skilled specialists in amino acid analysis, we are able to offer you professional assistance throughout each amino acid testing project.
Standard analysis of the amino acids (AAs) content in food can be conducted in three steps:
Each sample can be subjected to 3 different hydrolysis approaches: HCl acid hydrolysis for determination of the most abundant amino acids; performic acid oxidation for sulfur-containing amino acids (methionine and cysteine) analysis following the HCl acid hydrolysis; and alkaline hydrolysis for tryptophan assessment. We also provide microwave hydrolysis, which can be implemented with all these three hydrolysis methods and result in improved control of hydrolysis conditions with better accuracy, reproducibility, efficiency, and robustness.
Lifeasible offers many different chromatographic techniques to help you separate and quantify amino acids. These techniques include paper chromatography, thin-layer chromatography (TLC), ion-exchange chromatography, reversed-phase chromatography, gas-liquid chromatography, and so on.
Amino acids, with the exception of tryptophan, tyrosine, and phenylalanine, cannot be easily detected using absorbance. Consequently, amino acids must be derivatized post-column (for ion-exchange chromatography) or pre-column (for reversed-phase chromatography). Ninhydrin and o-phthaldialdehyde (OPA) are two of the most commonly used derivatizing reagents for post-column. 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC), phenylisothiocyanate (PITC), and 9-fluorenylmethyl chloroformate (FMOC) are widely used for pre-column.
The separated AAs can be quantified by various detection vertices through absorbance or fluorescence of the derivatives according to the corresponding calibration curves (Figure 2).
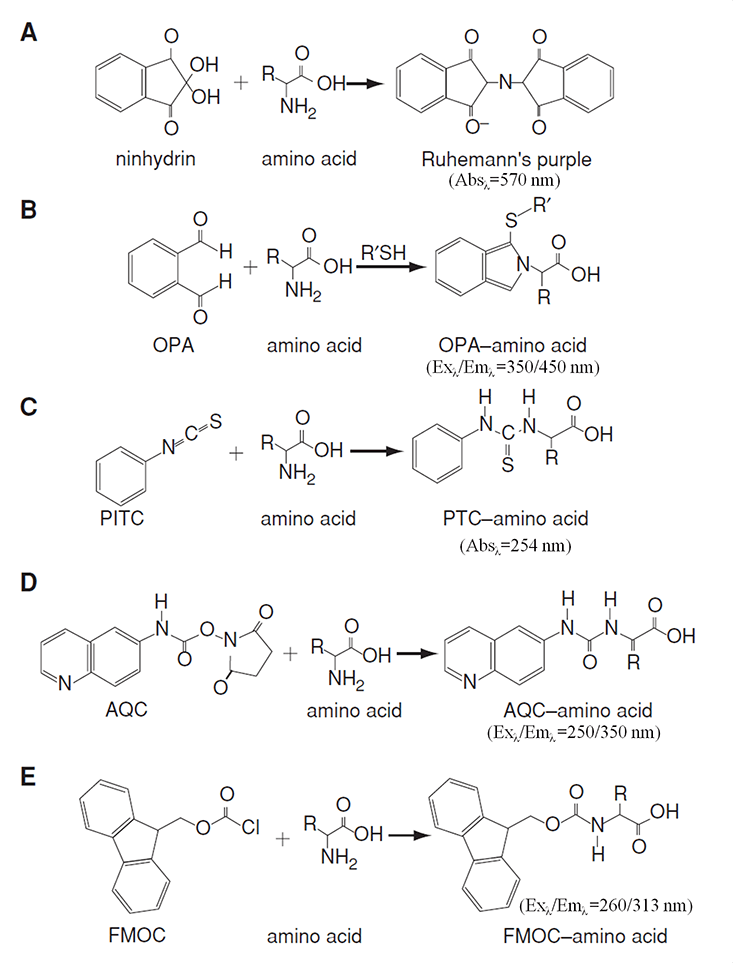 Figure 2. Derivatization reactions and their detections for amino acid analysis (Rutherfurd and Gilani, 2009)
Figure 2. Derivatization reactions and their detections for amino acid analysis (Rutherfurd and Gilani, 2009)
Why choose Lifeasible?
Would you like to learn more about our amino acid testing service? Please feel free to contact us. We will be more than happy to discuss further all the possibilities and help you achieve your goal.
Table 1 List of natural amino acids and their related compounds
| Common (natural) amino acids | Amino acid related compounds |
| Aspartic acid | Phosphoserine |
| Threonine | Taurine |
| Serine | Phosphoethanolamine |
| Asparagine | Hydroxyproline |
| Glutamic acid | Sarcosine |
| Glutamine | α-aminoadipic acid |
| Cysteine | Citrulline |
| Proline | α-aminobutyric acid |
| Glycine | Homocitrulline |
| Alanine | Cystathionine |
| Valine | β-alanine |
| Cystine | β-aminobutyric acid |
| Methionine | Homocystine |
| Isoleucine | γ-aminobutyric acid (GABA) |
| Leucine | Ethanolamine |
| Tyrosine | Hydroxylysine |
| Phenylalanine | Ornithine |
| Lysine | 1-Methylhistidine |
| Histidine | 3-Methylhistidine |
| Tryptophan | Anserine |
| Arginine | Carnosine |
Reference