Proteins are an important source of energy. The average energy density of proteins is about 4 kcal/g or 17 kJ/g, which is comparable to carbohydrates. Proteins contain essential amino acids, such as lysine, tryptophan, methionine, leucine, isoleucine, and valine, which are essential to human health. Moreover, proteins play important roles in regulating human body functions, such as catalyze biological activities, and transport nutrients and other biochemical compounds across cellular membranes. In addition, proteins are major structural components of many natural foods, and can affect the texture, appearance, or stability of food products. Thus, it is important to develop methods for analysis of the identification, concentration, structure, and functional properties of proteins in foods, in order to meet labeling requirements for food quality controls.
Lifeasible has rich experience and cutting-edge facilities in protein analysis of foods. We have developed an advanced platform providing multiple methods for protein analysis in foods, which include, but not limited to:
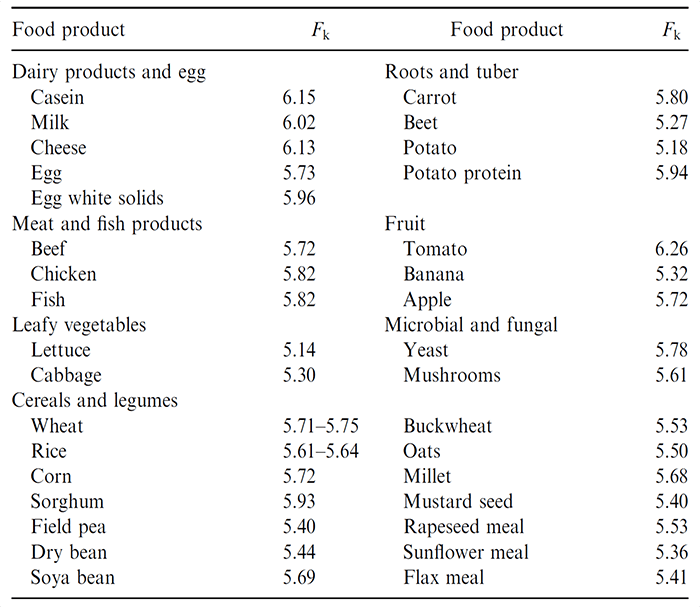
The most commonly used biochemical methods to measure protein content are Kjeldahl method and enhanced Dumas method. The protein concentration is calculated by nitrogen content multiplying a conversion factor (Fk), which varies in different foods (Table 1).
- Kjeldahl method. This method requires three steps: digestion, distillation, and titration. Digestion converts nitrogen in the food into ammonia, and other organic matter to CO2 and H2O by heating (370ºC to 400ºC) in the presence of sulfuric acid, anhydrous sodium sulfate, and a catalyst. Ammonia is separated by raising the pH with sodium hydroxide, which turns ammonium ions into ammonia gas, which is then distilled into a trapping solution with hydrochloric acid. The nitrogen content is then evaluated by titration of the ammonium borate formed with standard hydrochloric acid.
- Enhanced Dumas method. The combustion method involves burning a sample with a known mass in an oxygen-rich atmosphere at a high temperature (about 900 °C). The combustion products—mainly CO2, H2O, NO2, and N2—are collected, then the CO2 and H2O are removed by passing through special columns, and the NO2 is converted to N2 by passing over hot copper. The nitrogen content is then measured by thermal conductivity.
Table 1. Nitrogen-protein conversion (Fk) factors for selected food protein sources (Owusu-Apenten, 2002)
- Spectrophotometric methods
At Lifeasible, a variety of spectrophotometric methods are available to determine protein content in foods, including:
- Direct spectrometry measurement at 280 nm. The aromatic amino acids such as tryptophan and tyrosine absorb ultraviolet light at 280 nm, which is usually used to determine the concentration of protein in foods.
- Biuret method. This method is based on the binding of copper (II) to a peptide bond in protein molecules under alkaline conditions, producing a violet-purplish color complex with absorbance at 540 nm.
- Lowery method. The Lowry method combines the biuret reagent with another reagent (the Folin-Ciocalteau phenol reagent) which reacts with tyrosine and tryptophanresidues in proteins, resulting in a bluish color product with strong absorbance at 500 - 750 nm.
- Dye binding method. This method relies on the principle that the proteins with positive charges can form an insoluble complex with the negatively charged dye due to electrostatic attraction. The excess and unbound dye remains soluble and can be determined by measuring its absorbance
- Turbimetric method. The reaction of protein molecules with certain chemicals (e.g.,trichloroacetic acid), may lead to protein precipitations, which can be detected and analyzed by the turbidity of the solution.
- Bradford method. This method is based on the principle that Coomassie G-250 dye can bind to proteins through basic amino acids (primarily arginine, lysine, and histidine), leading to a color change from reddish-brown to bright blue, with an absorbance peak at 595 nm.
- IR spectrophotometry. Infrared spectroscopy is based on absorption of a wavelength of infrared radiation specific for the peptide bond. It is a fast, simple, and non-destructive method for the determination of protein in cereal products.
- Nuclear magnetic resonance (NMR) spectroscopy: NMR spectroscopy can be used to determine the total protein concentration of foods by measuring the area under a peak in an NMR chemical shift spectra that corresponds to the protein fraction.
- Chromatography, electrophoresis, and immunology
Specific protein components in food and food products can be quantified by chromatography (e.g., HPLC and GC), electrophoresis, immunology, or a combination of these techniques. As the most powerful analytical techniques for the analysis of specific protein components in foods, chromatographic methods are based on the partition coefficients, polarities or sizes of proteins by passing the solution to be analyzed. The separation of proteins in an electric field relies on the basis of their charge density. Immunological methods such as immunoelectrodiffusion and enzyme-linked immunosorbent assay are based on the interaction between an antigen and its corresponding antibody. These are highly specific and sensitive means to identify and quantify minor protein components in complex matrices.
Lifeasible also offers a variety of physical methods for protein content measurement in foods. The physical properties, such as density, refractive index, and light or ultrasonic scattering, are proportional to the concentration of protein in food and food products.
Our state-of-the-art facilities are powerful for determination of protein values. We can provide accurate protein analysis to ensure compliance with all regulatory requirements concerning nutritional declarations, and to avoid extra cost or delays in bringing your products to market. Welcome to contact us for more information.
Reference
-
Owusu-Apenten, R.k.. Food protein analysis. Quantitative effects on processing. Marcel Dekker, Inc, 270 Madison Avenue, New York. 2002.
For research or industrial raw materials, not for personal medical use!

