Surface Plasmon Resonance (SPR) spectroscopy is a binding analysis technology used for the characterization of molecular interactions. Since its first application in the detection of protein-protein interactions, the SPR technology has become a popular tool for the quantitative analysis of biomolecular interactions.
Surface plasmon resonance refers to the physical phenomenon when polarized light hits a metal film at a certain incidence angle (ie., the resonance angle), it will be absorbed by the electrons within the metal film and cause them to resonant. As the surface plasmon resonance is very sensitive to the surrounding environment, this principle can be utilized for the detection of molecular interactions by measuring the resonant oscillation changes. Specifically, the “probe” molecules are immobilized on the surface of a metal film, and the “bait” molecules will pass through a channel and be exposed to the film. In the meantime, polarized light keeps exciting the surface plasmon in the metal film and the surface plasmon signal is collected by a detector. Upon binding of “bait” molecules to the “probe” molecules, there will be a shift of the reflectivity curve (Figure 1).
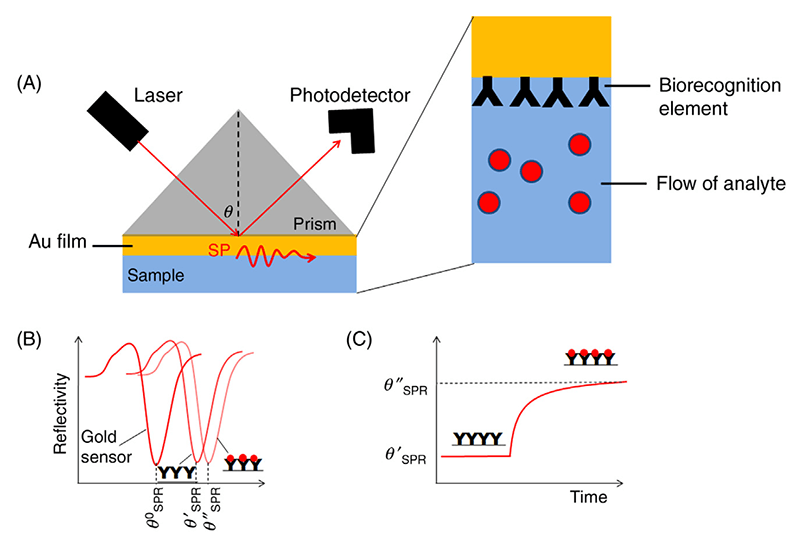 Figure 1. Schematic representation of the SPR principle (Celina M. Miyazaki 2017) (A) Experimental set up of a standard SPR assay. (B) The reflective curve shifts after binding of “bait” proteins. (C) A diagram showing the real-time monitoring of the protein-protein interaction event.
Figure 1. Schematic representation of the SPR principle (Celina M. Miyazaki 2017) (A) Experimental set up of a standard SPR assay. (B) The reflective curve shifts after binding of “bait” proteins. (C) A diagram showing the real-time monitoring of the protein-protein interaction event.
Over the past two decades, the SPR spectroscopy technique has become increasingly popular, both for academic researches and pharmaceutical industries. Comparing with other protein affinity testing approaches, the SPR technique has multiple distinct advantages:
Lifeasible is an outstanding plant biotechnology company with years of experience in the expression and characterization of plant proteins. We provide high quality SPR services for the detection and quantification of plant biomolecule interactions. Our well-established SPR platform has both high-standard working capacity and the feasibility to adapt to the various requests from different experimental objectives. Moreover, we offer featured one-stop services that covers every step of your project, from protein expression and purification, to the performance of SPR spectrometry and data reporting. Our experienced experts and scientist will also work closely with you on experimental design, trouble shooting, project progress updating and data interpretation.
Reference