Lifeasible facilitates the application of gene editing technology in plant protection and provides gene knock-in or overexpression services to facilitate the investigation and research of gene editing in plant protection.
Knock-in or overexpression of genes with resistance to pests, diseases, and adverse environments in plants can effectively achieve plant protection. Different metabolites in plants play different roles in protecting plants. Long-lasting plant protection can be achieved by gene editing to induce the production of these plant protection-related metabolites in target plants. In addition, plant protection can also be achieved by expressing plant-protective metabolites from microorganisms. Transgenic cotton that expresses Bt (Bacillus thuringiensis) proteins is a good example. It is also possible to achieve resistance to adverse environments by knock-in or overexpression of genes. For example, overexpression of OsERF71 enhances drought tolerance in transgenic rice, and overexpressing the AnnSp2 gene in tomato genotypes shows tolerance to drought and salinity stresses. In plants, the commonly used method for gene knock-in and overexpression is based on CRISPR/Cas.
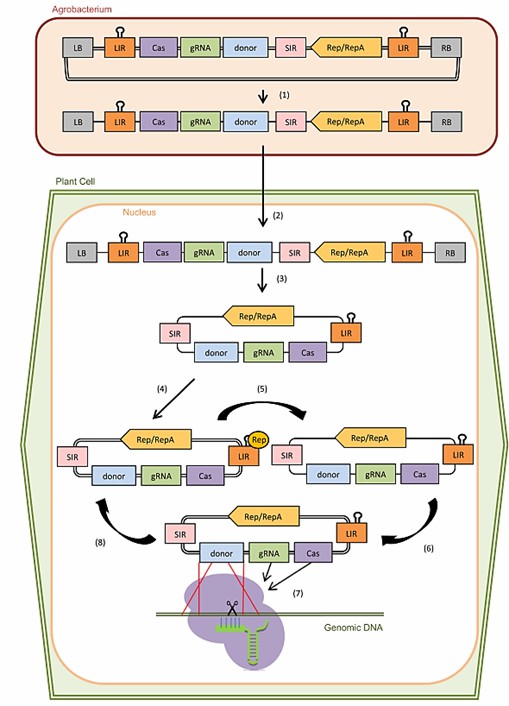 Fig. 1 CRISPR-induced gene knock-in mediated by geminivirus-derived replicons in plant cells (Collonnier et al., 2017).
Fig. 1 CRISPR-induced gene knock-in mediated by geminivirus-derived replicons in plant cells (Collonnier et al., 2017).
Lifeasible provides professional services to facilitate the knock-in and overexpression of genes. We are well-positioned to address the knock-in and overexpression issues. We mainly provide knock-in and overexpression in plants.
We offer to knock in or overexpression plant protection-related genes in target plants, including biocontrol agents-related genes, plant stress resistance-related genes, and herbicide-resistant genes. This service aims to enhance the plant's resistance to diseases, abiotic stresses, and herbicides. We offer to knock in or overexpression biocontrol agents-related or bioactive substances-related genes in microorganisms. This service aims to construct engineered microorganisms for plant protection. Then, we offer knock-in of strong promoter genes for overexpression. We also provide knock-in and overexpression services for unknown functional genes to help explore the plant protection function of the genes.
Knock-in or overexpression genes in target plants
We mainly perform gene knock-in based on CRISPR/Cas9 technology. Overexpression of genes can be achieved by constructing overexpression vectors and then transforming them into plant cells. We mainly use Agrobacterium-mediated transformation to achieve overexpression services.
Knock-in or overexpression genes in microorganisms
We achieve gene knock-in or gene overexpression in microorganisms by CRISPR/Cas technology and homologous recombination. We use CRISPR/Cas9 and CRISPR/Cas12 for precise knock-in, and we use the mariner transposon-base expression cassette integration method for random knock-in.
Effective ways to improve knock-in efficiency
Targeted double-strand break (DSB) repair by non-homologous end-joining (NHEJ)-mediated knockout genes is easier to master, while homology-directed repair (HDR)-mediated knock-in genes remain challenging to perform efficiently in higher plants. We offer the suppression of NHEJ recombinant cofactor and overexpression of HDR enhancer to improve HDR efficiency in gene knock-in.
Lifeasible provides knock-in and overexpression services for target genes related to plant protection using gene editing. In addition, we can also achieve overexpress for existing genes using non-gene editing methods, including saRNA and CRISPR SAM (synergistic activation mediator) methods. Please contact us for professional services.
References