Redefining the Sensory Landscape of Natural Sweeteners
Sweet proteins, with their natural origin, zero glycemic index, and sweetness thousands of times greater than sucrose, have become an ideal alternative in the food industry. However, the commercialization of sweet proteins faces significant challenges, primarily due to the scarcity of natural resources, the complexity of protein folding in heterologous expression, and maintaining stability during processing.
Lifeasible offers a comprehensive sweet protein service platform, integrating plant biotechnology and advanced analytical techniques to assist clients in the identification, expression, purification, and validation of highly active proteins. Whether you are in the early stages of research and development or seeking commercialization, our expert team can provide extensive technical support, from laboratory prototypes to market-ready solutions.

For many industrial applications, plants themselves offer the most scalable and cost-effective bio-reactors. Our sweet protein transgenic plants development service focuses on the stable integration of sweet protein genes into high-yield crop systems.
We offer transformation services for a wide variety of species, including model plants like Arabidopsis and Nicotiana benthamiana, as well as food crops like rice (Oryza sativa), maize (Zea mays), and tomato (Solanum lycopersicum).
Our laboratory utilizes state-of-the-art Agrobacterium-mediated transformation, particle bombardment (gene gun), and floral dip methods to ensure high-efficiency gene delivery. We maintain an extensive library of Agrobacterium tumefaciens strains (e.g., LBA4404, GV3101, EHA105) tailored to specific host requirements.
We construct sophisticated expression vectors featuring tissue-specific promoters (such as seed-specific or fruit-specific promoters) to concentrate sweet protein accumulation where it is easiest to harvest.
Beyond initial transformation, we provide T0 to T3 generation screening, copy number analysis (via qPCR/ddPCR), and stability testing to ensure the "sweet" phenotype is heritable and consistent.
When rapid production cycles and controlled environments are required, microbial systems provide a robust alternative. Our sweet protein production strains development service is dedicated to engineering microorganisms for high-titer protein secretion.
We develop optimized strains of Escherichia coli, Pichia pastoris, and Saccharomyces cerevisiae. For proteins requiring complex disulfide bond formation (like Brazzein), we utilize specialized yeast strains with modified secretory pathways.
We don't just provide the strain; we optimize the recipe. This includes codon optimization tailored to the specific microbial host, selection of high-strength inducible promoters (like AOX1 or GAL1), and optimization of media components to maximize yield while minimizing off-flavors.
Our strains are engineered for extracellular secretion whenever possible, significantly reducing the complexity and cost of subsequent purification steps.
All developed strains are documented for biosafety compliance, ensuring the final protein product is suitable for food-grade applications.

The botanical world still hides many undiscovered sweet-tasting molecules. We utilize bioinformatics and comparative genomics to identify novel sweet protein candidates.
Identifying potential sweet proteins in uncharacterized tropical flora based on structural similarities to known families (e.g., Thaumatin-like proteins or TLP family).
Using molecular docking to simulate how candidate proteins interact with the human sweetness receptors T1R2 and T1R3.
Sweet protein testing is the cornerstone of our quality assurance. Because sweetness is a biological response, physical concentration does not always correlate with sensory impact.
Utilizing SDS-PAGE, Western Blotting, and High-Performance Liquid Chromatography (HPLC) to ensure >95% purity.
Circular Dichroism (CD) and Mass Spectrometry (LC-MS/MS) to confirm the protein is correctly folded and stable.
Determining the "Sucrose Equivalent Value" (SEV). We quantify how many times sweeter the protein is compared to a standard 5% or 10% sucrose solution.
Evaluating protein performance across various pH levels and temperatures (e.g., pasteurization conditions) to ensure functionality in beverages and dairy.

Our workflow is designed to be transparent, iterative, and data-driven, ensuring that every milligram of protein produced meets the highest standards of the biotech industry.
![]()
Project Consultation & Design
We define the target protein based on your application (heat stability vs. highest potency) and select the optimal expression host (Plant vs. Microbe).
![]()
Gene Synthesis & Vector Construction
Designing high-efficiency expression vectors with specialized promoters, signal peptides for secretion, or vacuolar accumulation tags.
![]()
Pilot Expression & Optimization
Small-scale trial to assess yield and protein solubility. We adjust pH, temperature, and induction parameters to maximize "sweet-active" protein fractions.
![]()
Scale-up & Purification
Transitioning to large-scale cultivation or greenhouse production. We employ multi-step purification strategies, including Ion Exchange Chromatography (IEX) and Size Exclusion Chromatography (SEC).
![]()
Quality Control & Sweet Protein Testing
A rigorous battery of tests to confirm protein sequence, folding, and microbial purity.
![]()
Sensory Validation & Reporting
Detailed analysis of sweetness intensity and a comprehensive technical report suitable for regulatory filings or patent applications
To ensure the success of our analysis or expression services, please refer to the following guidelines for submitting samples or genetic data.
| Sample Type | Minimum Requirement | Recommended Condition |
| Genetic Sequences | Digital sequence (FASTA) | Accession number or synthesized plasmid |
| Purified Protein | 5 mg - 50 mg | Lyophilized or in volatile buffer (Ammonium acetate) |
| Plant Tissue (for extraction) | 50 g (Fresh Weight) | Flash-frozen in liquid nitrogen, shipped on dry ice |
| Cell Lysates | 10 mL | Frozen with protease inhibitor cocktail |
| Reference Compounds | 10 mg | Analytical grade (if specific benchmarking is required) |
Plant-Centric Bioengineering
Unlike generalist protein manufacturers, we possess deep expertise in plant physiology, allowing us to overcome unique challenges like cell-wall interference and secondary metabolite contamination.
End-to-End Solutions
From initial gene mining to final sweet protein testing, we manage the entire pipeline.
Customizable Expression Pathways
Whether you require the rapid turnaround of Sweet Protein Production Strains Development or the massive scalability of Sweet Protein Transgenic Plants Development, we offer the right platform for your budget and timeline.
Publication & Regulatory Ready
We provide the data depth required for both high-impact academic journals and regulatory dossiers (GRAS, EFSA).
Unlock the potential of nature's most powerful sweeteners. Whether you are an ingredient innovator or a beverage manufacturer, our Sweet Protein Service provides the scientific foundation to replace sugar without compromising on taste.
Contact our technical team today for a consultation on your sweet protein expression or testing needs.
Sweet proteins are a unique class of non-terpenoid, non-saccharide molecules synthesized by specific plant species. Unlike small-molecule artificial sweeteners (like Aspartame or Sucralose), sweet proteins are large macromolecules composed of amino acids. When consumed, they are digested into standard amino acids, leaving no metabolic residue and contributing negligible calories.
The most famous examples include:
The human tongue perceives sweetness through a G-protein coupled receptor (GPCR) complex known as T1R2/T1R3. While sugar molecules bind to small pockets within this receptor, sweet proteins are so large that they interact with the external "Venus Flytrap" domain (VFTD) of the receptor. This high-affinity binding explains why sweet proteins are effective at micromolar concentrations, whereas sucrose requires millimolar concentrations to elicit the same response.
The sweetness of a protein is entirely dependent on its three-dimensional conformation. If a sweet protein like Monellin is heated to the point of denaturation, it loses its taste entirely, even though the amino acid sequence remains unchanged. Therefore, sweet protein testing is not just about measuring quantity; it is about measuring bio-activity.
Key analytical components include:
The global demand for sugar alternatives cannot be met by wild harvesting. Plant biotechnology offers two primary solutions:
It depends on the specific protein. Brazzein is remarkably heat-stable due to its four disulfide bonds and can withstand boiling and industrial pasteurization. Thaumatin is moderately stable at acidic pH but can lose potency at neutral pH and high heat. Monellin is the most heat-sensitive. Our stability testing service helps you choose the right protein for your specific product matrix.
We use a two-tiered approach. First, we perform In Vitro Testing using receptor-binding assays to confirm the protein interacts with the T1R2/T1R3 receptor. Second, we conduct Sensory Panel Evaluation, where trained tasters compare the protein solution against a sucrose reference scale to determine the sweetness-to-weight ratio.
Yes. Sweet proteins are not carbohydrates. They do not raise blood glucose levels or trigger insulin secretion, making them an ideal sweetener for diabetic-friendly formulations.
The most common reason is misfolding. If the protein is produced in a system (like certain E. coli strains) that cannot form complex disulfide bonds, the protein will be biologically inactive. Our service includes "refolding optimization" and specialized Sweet Protein Production Strains Development to ensure the protein reaches its native, sweet-active state.
Thaumatin is widely approved as a sweetener and flavor enhancer (E957) in many regions, including the EU and USA. Others, like Brazzein and Miraculin, are currently in various stages of GRAS (Generally Recognized as Safe) notification or Novel Food approval. We provide the high-purity samples and characterization data necessary to support these regulatory applications.
Yes. We have specialized protocols for the expression of Miraculin in plant systems through our Sweet Protein Transgenic Plants Development Service. Our sweet protein testing for Miraculin involves specific pH-gradient sensory tests to quantify the conversion of citric acid (sour) into a sweet sensation.
Plant-based production (Molecular Farming) is generally more cost-effective at very large scales and carries a lower carbon footprint. Microbial production (Precision Fermentation) offers faster production cycles (days vs. months) and greater control over batch-to-batch consistency. We can help you determine the best path based on your specific volume requirements.

Creating Fragrant Corn with CRISPR/Cas9 Technology
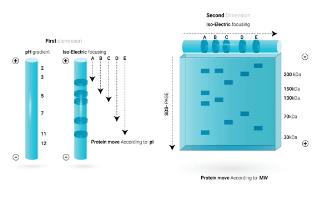
Separation of Proteins by Two-dimensional Gel Electrophoresis

Determining The Relative Molecular Mass of Proteins Using SDS-PAGE

Production of Secondary Metabolites By Large-scale Plant Cell Culture