Plant development is highly adapted to environmental conditions such as light, sunshine, and temperature. Despite this, plant body organs, such as inflorescences and flowers, develop robustly under different environmental conditions. How plant core organ development mechanisms adapt to environmental fluctuations to maintain robust development remains unclear, impacting the ability to create crops that are resilient to climate change.
On August 7, 2023, Nature Plants published online a research paper titled "A network of CLAVATA receptors buffers auxin-dependent meristem maintenance" by Zachary L. Nimchuk's team at the University of North Carolina at Chapel Hill. This study found that under different temperature environments, CLV3 signaling can regulate multiple auxin-dependent shoot growth and development processes and coordinate development by exerting opposite effects on different cell proliferation in the inflorescence meristems region.
Understanding how plants integrate different signals to maintain development in different environments is a key challenge. This study demonstrates that temperature and CLV signaling intersect to maintain multiple auxin-regulated IM functions in thermal environments. CLV3p plays a key role in the auxin-dependent flower primordium formation process, and its receptors CLV1 and CLV2/CRN both play a role in this process. Therefore, CLV3/CLE signaling can inhibit and promote cell proliferation in different IM regions through the same set of receptors. In addition, CLV1 seems to non-autonomously promote L1 auxin-responsive cells from meristem organizing center (OC) cells, but cannot promote CRN-expressing cells, indicating that there are significant spatial differences in the expression of CLEp receptors in the IM zone. However, CLV3p is widely diffused in the meristem and expressed in the central zone 3-5 cells away from the P0/P1 primordium, suggesting that it can directly affect the primordium. The study found that CLV3 inhibited the expression of CLE25 and ectopic CLE25 in the primordium, and the cle25 mutant did not enhance clv3 carpel number. Therefore, in addition to CLV3p, there is a transcriptional compensation mechanism for CLE25 in the nascent primordium, thereby partially compensating for the loss of CLV3, and the regulation of CLE25 affects the formation of flower primordia and the expansion of inflorescence primordia, but does not affect the regulation of flower stem cells. At the same time, CLE25 expressed from the CLV3 promoter can restore clv3 carpel number, indicating that there are temporal or spatial differences in CLE25 expression.
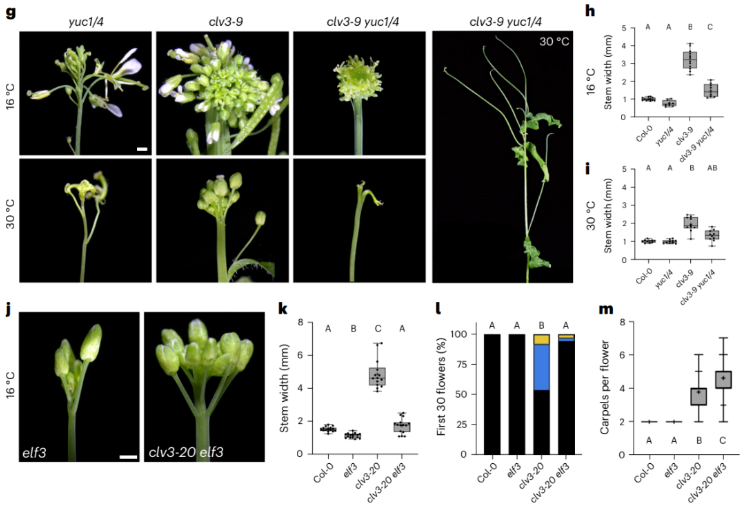
How CLEp signaling regulates auxin export is unclear. Reversal of clv1 primary inflorescence termination (PIT) and primordia defects caused by high temperature or ELF3 deletion, as well as repair of crn primordium defects by ectopic expression of YUC1, suggests that CLV signaling is not important for auxin perception, transport, or signaling. Because the crn primordium defect is not related to the ectopic expression of WUS, and the expression of WUS in clv1 plants that undergo PIT is not different from that in plants that do not undergo PIT, it is not known whether WUS mediates the auxin process of clv1/crn. Although IM size and auxin levels are inversely correlated, the PIT phenotype suggests a novel role for CLV1 in promoting auxin-dependent IM maintenance. Experimental data shows that thermal and ELF3 losses are not exactly equal. Both conditions suppressed PIT, floral primordium termination and IM phenotypes. However, heat suppressed clv carpel numbers, while deletion of ELF3 slightly increased them, suggesting different patterns in floral meristems (FM) and IM processes. Curiously, heat enhanced the primordium growth defect of clv3 yuc1/4 mutants, similar to but more severe than the clv2 yuc1/yuc4 primordium defect, suggesting that heat itself may negatively impact auxin function.
Taken together, this work reveals that temperature and CLV signaling intersect to maintain multiple auxin-regulated IM functions in temperature environments. CLV3 signaling is also essential in auxin-dependent floral primordia formation, a function partially obscured by inflorescence clustering and heat-induced auxin biosynthesis. The CLAVATA signaling pathway is dissociable for stem cell regulation and primordium formation, but is also sensitive to temperature and auxin levels. In addition, the authors discovered that the CLV3 receptor CLAVATA1 has a novel role in auxin-dependent meristem maintenance in colder environments.
Reference:
John, A., Smith, E.S., Jones, D.S. et al. A network of CLAVATA receptors buffers auxin-dependent meristem maintenance. Nat. Plants 9, 1306–1317 (2023).