Citrus canker caused by Xanthomonas citri subsp. citri (Xcc) causes severe yield, quality, and economic losses to citrus production worldwide. Xcc encodes the pathogenic factor PthA4, which enters the nucleus of plant cells and activates the canker susceptibility gene LOB1 by binding to the effector binding element of the promoter region, thereby inducing the expression of downstream genes and leading to typical canker symptoms, including hypertrophy and hyperplasia.
Recently, CRISPR/Cas-mediated genome editing of the LOB1 promoter or coding region confers resistance to Xcc in citrus. However, the citrus produced by the CRISPR genome editing method was genetically modified. The use of GM crops in production faces many challenges due to regulatory and public perception concerns. Consequently, despite enormous efforts and superior disease resistance, none of the citrus produced through biotechnological methods has been registered and commercialized.
On July 5, 2023, Nature Communication published online the latest research progress "Generation of the transgene-free canker-resistant Citrus sinensis using Cas12a/crRNA ribonucleoprotein in the T0 generation" by Wang Nian's team at the University of Florida. In this article, the researchers used Cas12a/crRNA RNP to transform protoplasts, and obtained a non-transgenic canker-resistant citrus line of the T0 generation within 10 months, and the biallelic/homozygous mutation rate in the mutant plants was 97.4%, and no off-target mutations were detected. This study provides a sustainable and effective solution for citrus canker control and provides an effective transgene-free genome editing strategy for citrus and other crops.
The researchers first used Cas12a/crRNA RNP to digest the CsPDS gene in vitro to evaluate the editing efficiency of RNP. The results showed that both ErCas12a and LbCas12aU could efficiently digest DNA fragments, and the in vitro digestion efficiency of LbCas12aU was slightly higher than that of ErCas12a.
Next, the researchers began to use the PEG-induced method to transform LbCas12aU/crRNA and ErCas12a/crRNA RNP into citrus protoplasts to edit CsPDS and regenerate them. Sequencing results showed that the mutation rates of LbCas12aU/crRNA and ErCas12a/crRNA were 14.3% and 16.7%, respectively. And the 58 albino embryos transformed with ErCas12a included 56 homozygous mutants, 1 biallelic mutant and 1 chimeric mutant. Taken together, the researchers concluded that both LbCas12aU/crRNA and ErCas12a/crRNA RNP transformation of embryogenic citrus protoplasts can efficiently generate biallelic/homozygous CsPDS mutations for citrus.
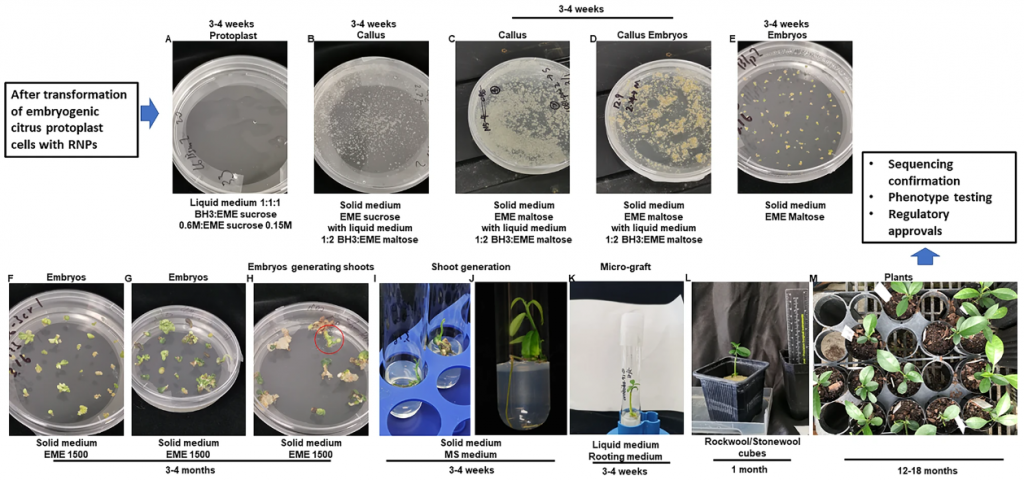
After testing the feasibility of Cas12a/crRNA RNP, the researchers began to edit the CsLOB1 gene in vivo. After comparison, LbCas12aU showed excellent activity, so LbCas12aU was used for editing, and finally a total of 42 regenerated plants were obtained, and 39 lines survived in the greenhouse, of which 38 lines were homozygous (8 lines)/biallelic. As expected from RNP-mediated genome editing, analysis of whole-genome sequencing data revealed that all 12 edited lines were free of foreign genes. In addition, the researchers also investigated whether the non-transgenic lines contained off-target mutations. After genome-wide sequencing analysis, the sequences of CsLOB2 and CsLOB3 in the 12 edited lines were the same as the corresponding sequences of the wild type, and no off-targets occurred.
After obtaining the mutants, the researchers evaluated the cslob1 mutants. The results showed that 32 out of 38 biallelic/homozygous cslob1 mutants were phenotypically similar to wild-type cslob1 mutants. However, six lines had narrower leaves (which the researchers explained may be due to somaclonal variation during tissue culture).
As expected, infection of Xcc with biallelic/homozygous cslob1 mutants did not cause any canker symptoms. Mutations in the CsLOB1 gene abolished the typical leaf tissue hypertrophy and hyperplasia caused by Xcc. Significant differences in Xcc titers were observed between wild type and cslob1 mutants. In addition, the researchers performed foliar sprays on wild-type and cslob1 mutants to mimic natural infection with Xcc. Canker symptoms were observed around wounds in wild type but not in cslob1 mutants. Likewise, Xcc was significantly lower in cslob1 mutants than wild type.
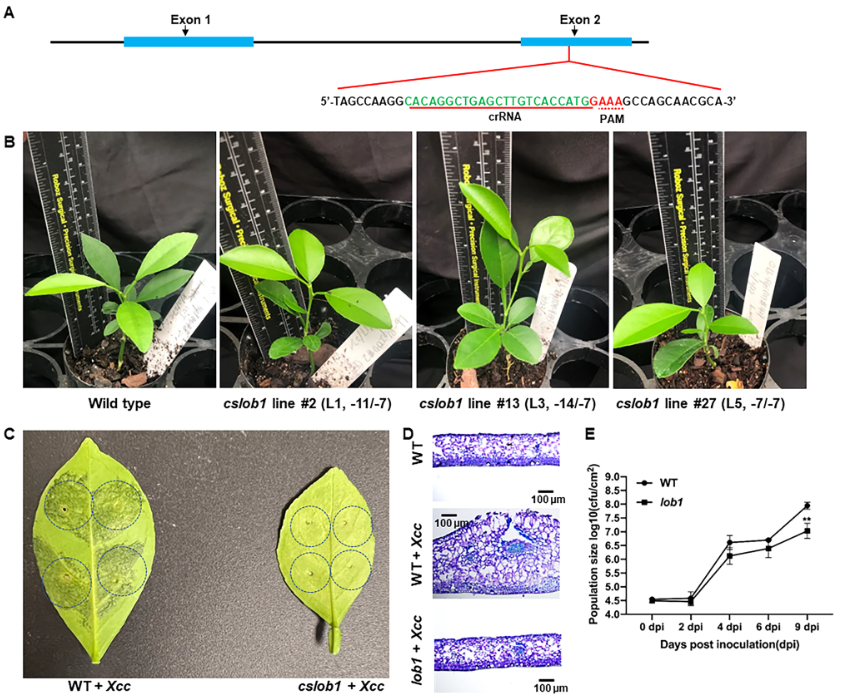
Taken together, this study produced transgenic-free canker-resistant citrus lines that are under evaluation, providing a sustainable and effective solution for controlling citrus canker.
At the same time, the use of RNP technology applied by the researchers in this article to efficiently edit the genome of citrus without transgenes is expected to have a significant impact on the genetic improvement of citrus varieties.
Reference:
Su, H., Wang, Y., Xu, J. et al. Generation of the transgene-free canker-resistant Citrus sinensis using Cas12a/crRNA ribonucleoprotein in the T0 generation. Nat Commun. 14, 3957 (2023).