The widespread adoption of genetically modified crops has revolutionized agriculture. However, resistance of agricultural pests to insecticidal proteins poses an ongoing challenge to maintaining crop productivity, and there is an urgent need for new proteins to replace those used in existing transgenic traits. Pore-forming proteins (PFPs) are a major class of soluble proteins that oligomerize into rings and undergo large irreversible conformational changes to become transmembrane assemblies and breach target cell membranes. The resulting uncontrolled influx/efflux of ions in the pores can cause severe osmotic imbalance and toxicity, ultimately leading to cell death.
PFPs from Bacillus thuringiensis (Bt) have been the basis for genetically modified insect-resistant crops and have been used by growers around the world for nearly three decades. The insect resistance provided by PFP is very effective and has environmental and economic benefits. One of the most destructive pests affecting corn production in North America and Europe, causing significant yield losses, is the coleopteran western corn rootworm (WCR; Diabrotica virgifera virgifera). Since the early 2000s, genetically modified corn expressing Bt proteins from the Cry3 class (mCry3Aa, Cry3Bb1, and eCry3.1Ab) has allowed this pest to be effectively controlled in North America. However, these proteins have experienced reduced efficacy as WCR develops resistance in field populations. As the discovery of useful Bt-derived insecticidal proteins becomes increasingly difficult, it is urgent to find novel proteins with high activity and new modes/sites of action from non-Bt organisms.
On July 13, 2023, Nature communications published a research paper titled "Structural journey of an insecticidal protein against western corn rootworm" online. The authors identified Mpf2Ba1, an insecticidal protein active on WCR, and performed a comprehensive structure-function characterization of PFPs from non-Bt organisms, revealing key molecular mechanisms of pore formation.
The authors first screened the biological activity of artificial feed for WCR larvae, and isolated Mpf2Ba1 from Pseudomonas montessorii through a multi-step purification process. Mpf2Ba1 is a 53.2 kDa protein that shows good insecticidal effect on WCR. The authors then solved the X-ray structure of Mpf2Ba1 and showed how the N-terminal residues inhibit the association of the soluble form of the monomer.
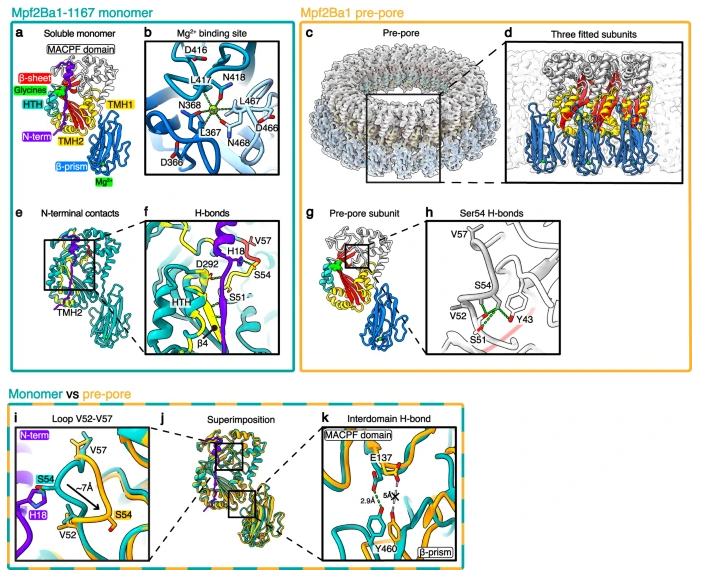
The authors then demonstrated that proteolysis in the presence of intestinal fluid activates monomer self-assembly into pre-pores and reconstructed the pre-pore by cryo-EM at 3.1 Å resolution. Subtle but significant changes between monomers and pre-pore subunits reveal molecular mechanisms related to target recognition and activation/oligomerization. Thermal-induced pre-pore-to-pore transition allowed us to resolve the 21-mer pore cryo-EM structure at 2.6 Å resolution, showing significant conformational changes in the pore subunits and their HTH-mediated intersubunit stability.
In summary, the authors identified and characterized the non-Bt insecticidal protein Mpf2Ba1 and elucidated the molecular mechanism of how it effectively kills the devastating corn pest WCR by forming pores in the midgut membrane. The pore's binding site is different from the insecticidal proteins currently used in commercial genetically modified corn hybrids. WCR larvae feeding on Mpf2Ba1-protected maize ingest Mpf2Ba1 monomers expressed by plant root tissue. Soluble monomers reach the larval midgut and are activated by intestinal proteases before/while binding to a not-yet-identified receptor (Rc) on target cell and oligomerize into pre-pores. The pre-pore transforms into an irreversible transmembrane pore, allowing uncontrolled ion flow across the plasma membrane, disrupting cell function and causing larval starvation.
|
Cat# |
Product Name | Size | Price |
| cry3B-01B | Recombinant Bacillus thuringiensis cry3B Protein | 1mg | $998 |
| cry1ab-02B | Recombinant Bacillus thuringiensis cry1ab Protein | 1mg | $998 |
| Vip3A-03B | Recombinant Bacillus thuringiensis Vip3A Protein | 1mg | $998 |
| cry1F-04B | Recombinant Bacillus thuringiensis Cry1F Protein | 1mg | $998 |
| cry2Ab-05B | Recombinant Bacillus thuringiensis cry2Ab Protein | 1mg | $998 |
| Vip3Aa19-06B | Recombinant Bacillus thuringiensis Vip3Aa19 Protein | 1mg | $998 |
| Cry1F-1-07B | Recombinant Bacillus thuringiensis Cry1F-1 Protein | 1mg | $998 |
| Cry1F-2-08B | Recombinant Bacillus thuringiensis Cry1F-2 Protein | 1mg |
$998 |
| Cry1A.105-09B | Recombinant Bacillus thuringiensis Cry1A.105 Protein | 1mg | $998 |
| Cry1Ia-10B | Recombinant Bacillus thuringiensis Cry1Ia Protein | 1mg | $998 |
Reference:
Marini, G., et al. Structural journey of an insecticidal protein against western corn rootworm. Nat Commun. 2023, 14: 4171.