Plant transformation technology refers to a biotechnological approach that introduces exogenous DNA into the plant genome to achieve gene expression or genetic modification. Its core goal is to precisely regulate plant gene function, thereby improving crop stress resistance, yield, or added value, promoting agricultural modernization and the development of the biopharmaceutical industry. Since the 1980s, this technology has gradually evolved from laboratory exploration to large-scale application, becoming a critical bridge between basic research and industrial practice.
This article will systematically analyze the scientific principles, technical framework, and application cases of plant transformation. Furthermore, it will explore its potential in crop improvement and medicinal plant development, taking into account quality control, regulatory compliance, and future trends. Through standardized process design and multidisciplinary integration, plant transformation technology is transitioning from experience-driven to engineering-based, providing a new path for global food security and sustainable development.
The totipotency of plant cells is the core foundation of plant transformation technology. According to plant physiology, plant cells can exhibit totipotency under specific conditions (such as in vitro culture), meaning that a single cell can differentiate into a complete plant. This property relies on the establishment of a plant regeneration system, including steps such as callus induction, somatic embryogenesis, and organogenesis. For example, after Agrobacterium-mediated transformation, exogenous genes are integrated into plant cells. Subsequently, tissue culture techniques (such as callus regeneration) enable rapid expansion of transgenic plants.
The fate of exogenous DNA in plant cells depends on its entry mechanism and subsequent integration process:
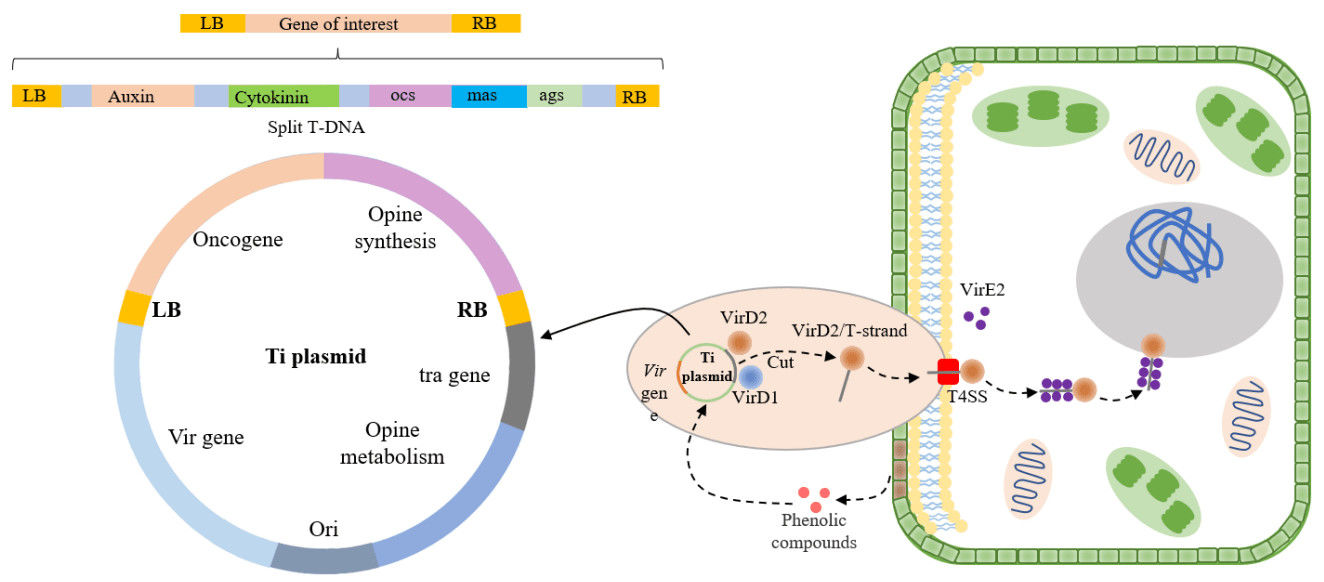 Fig. 1. T-DNA transfer and integration into the plant genome. (Su, et al., 2023)
Fig. 1. T-DNA transfer and integration into the plant genome. (Su, et al., 2023)
Agrobacterium tumefaciens is a natural model for plant transformation research. Its T-DNA-mediated transformation mechanism has been widely used in crop improvement. Agrobacterium recognizes signaling molecules (such as salicylic acid) released by plant wounds, activates genes in the Vir region, and integrates the T-DNA into the plant genome. This process not only reveals the molecular mechanisms of plant-microbe interactions but also provides a theoretical basis for the design of efficient transformation systems.
Plant transformation efficiency is limited by multiple factors:
A. tumefaciens regulates gene transfer through the Vir region (Virulence region) of its Ti plasmid. The Vir region contains a promoter and enhancer, and activation by inducers such as acetosyringone (AS) triggers the assembly of the Type IV secretion system (T4SS). The T4SS cleaves T-DNA from the Ti plasmid and packages it into a T-complex, composed of single-stranded T-DNA mediated by the VirD2 protein and double-stranded T-DNA mediated by the VirE2 protein. This complex is ultimately delivered to plant cells through the secretory pathway. The expression of genes in the Vir region depends on specific induction conditions, such as AS concentration and co-culture duration, to maximize T-DNA transfer efficiency.
T-DNA enters the plant cell nucleus via the Type IV secretion system of A. tumefaciens, where it subsequently unwinds and integrates into the host genome. This process involves both non-integrative transient expression of the T-DNA (e.g., expression of the reporter gene GFP) and stable integration. Integration site preference is influenced by the recipient cell type (e.g., leaf disc, protoplast) and T-DNA sequence (e.g., repetitive sequences). For example, transformation experiments with cotton Fusarium wilt pathogen showed that T-DNA integration efficiency was highest in IM medium at pH 5.3, and transformants required multiple subcultures to screen for stable expression.
T-DNA integration sites are typically concentrated in regions rich in repetitive sequences, such as telomeric repeats or near centromeres. Integration preferences vary significantly between plant species. For example, integration site distribution patterns differ between rice and cotton transformation systems, requiring verification through Southern blot analysis. Furthermore, integration efficiency is regulated by co-cultivation conditions (e.g., temperature, AS concentration) and recipient cell status (e.g., cell division activity).
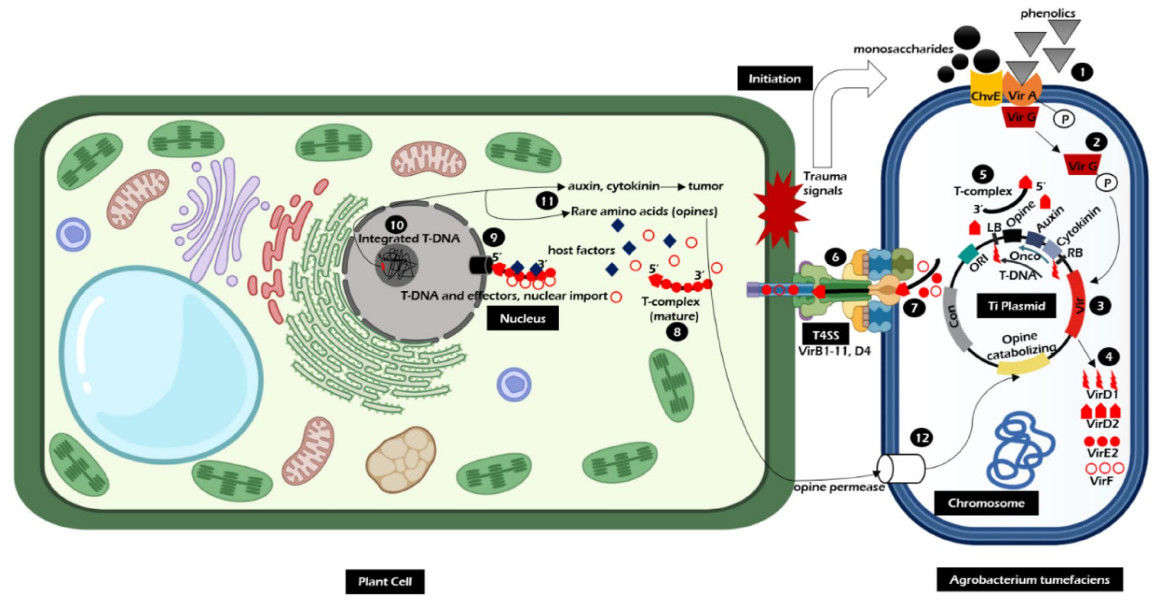 Fig. 2. Mechanism of action of Agrobacterium-mediated transformation. (Rahman, et al., 2024)
Fig. 2. Mechanism of action of Agrobacterium-mediated transformation. (Rahman, et al., 2024)
Gene gun technology utilizes gold or tungsten microparticles as carriers to coat DNA onto their surfaces. DNA coating is typically achieved through calcium phosphate precipitation or electroporation. The surface of the microparticles must maintain a neutral pH (5.0–6.0) to prevent DNA degradation. Particle diameter and surface charge balance are key factors influencing transformation efficiency.
The physical parameters of the gene gun (such as accelerating voltage and impact plate distance) must be optimized for different plant tissues. For example, wheat embryo transformation requires a voltage of 600 V and an impact plate distance of 5 cm to achieve efficient transformation. Furthermore, the co-culture time (typically 4–6 h) and the choice of selection medium (such as PDA containing hygromycin B) directly impact transformant purity.
Gene gun manipulation can cause cell damage and oxidative stress, which must be mitigated with antioxidants (such as MES buffer) and low temperature treatment (4°C). Maintaining the co-culture temperature at 24–28°C can reduce Agrobacterium contamination, while excessively high temperatures (>30°C) can inhibit T-DNA transfer.
Protoplast preparation relies on the synergistic action of pectinases and cellulases. The enzymatic digestion time (typically 30–60 min) must be strictly controlled to avoid excessive cell disruption. The pH (5.0–5.5) and ionic strength (e.g., Ca2+ concentration) of the digestion buffer significantly affect protoplast activity.
polyethylene glycol (PEG)-mediated DNA endocytosis relies on enhanced membrane fluidity, typically through the formation of transient pores via the PEG-Ca2+ complex. A balanced PEG concentration (10–20%) and Ca2+ concentration (0.5–1.0 M) are crucial; excessive concentrations can lead to cell death. Furthermore, endocytosis dynamics are limited by the cell cycle phase (e.g., G2/M phase) and the size of the exogenous gene (<10 kb).
Viral vectors (e.g., CMV and PVX) use replicons to drive transient expression of exogenous genes. For example, the CMV 35S promoter can drive efficient expression of reporter genes such as GFP, but homology conflicts with host genes must be avoided. Binary vectors (e.g., pGreenII) combining Agrobacterium and viral vectors can achieve synergistic effects of stable integration and transient expression.
Systemic spread of viral vectors relies on plant phloem transport, while Agrobacterium infiltration involves localized infection through wounds. The synergistic effect of these two approaches can expand the scope of transformation. For example, in tobacco, efficient transformation can be achieved by combining leaf disc transformation with Agrobacterium co-cultivation.
Electroporation uses an electric field to induce transient pores in the cell membrane and is suitable for protoplasts and tissue explants. The electric field strength (1000–2000 V/cm) and pulse duration (5–10 ms) need to be optimized for different plant species.
Nanocarriers (e.g., liposomes and gold nanoparticles) can improve DNA delivery efficiency and reduce cytotoxicity. For example, the use of gold nanoparticle-coated DNA in a gene gun has achieved efficient transformation of Setosphaeria zeae.
The pollen tube pathway utilizes the naturally occurring pathways of pollen tubes to deliver DNA into ovules and is suitable for monocotyledons. This method demonstrates high transformation efficiency in wheat and rice and requires no complex procedures.
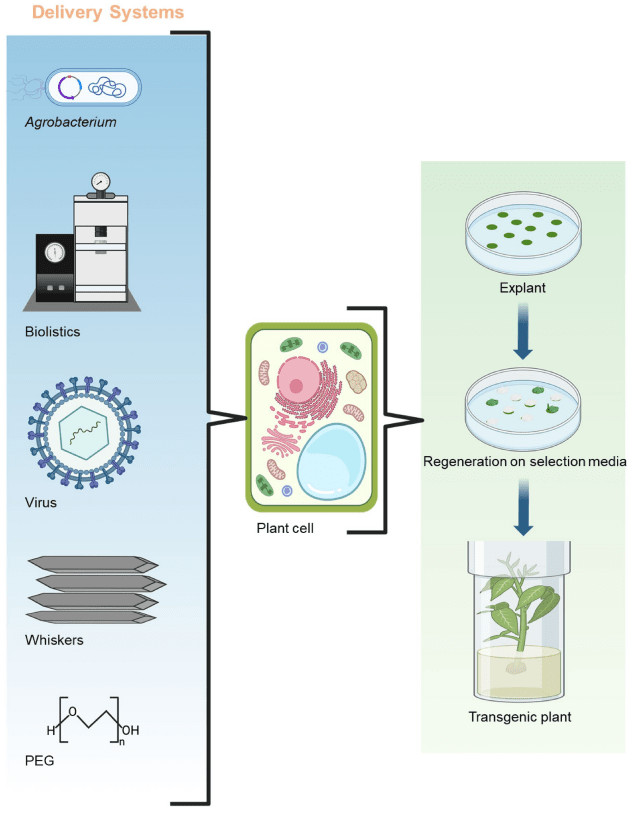 Fig. 3. delivery systems of plant genetic transformation. (Rahman, et al., 2024)
Fig. 3. delivery systems of plant genetic transformation. (Rahman, et al., 2024)
Binary vectors (e.g., pBI1J) drive T-DNA transfer via the Vir region of Agrobacterium, while super-binary vectors (e.g., pSB110) enhance gene expression through additional promoter modules (e.g., 35S, UBQ). Super-binary vectors offer advantages in complex gene networks (e.g., multi-gene editing).
Commonly used selectable markers include hygromycin B and kanamycin, and their selection should be based on the resistance profile of the recipient plant. Elimination strategies (e.g., homologous recombination) can remove selectable markers, reducing the environmental risks of transgenic plants.
Tissue-specific promoters (e.g., UBQ, PATAT1) can drive gene expression in specific tissues (e.g., roots, leaves, seeds). For example, the UBQ promoter achieves root-specific expression in Arabidopsis, while PATAT1 drives endosperm-specific expression in rice.
CRISPR/Cas9 achieves gene knockout/knockin through RNA-guided DNA cleavage and has been widely used in crops such as rice and wheat. The in vivo delivery efficiency of Cas9 protein can be optimized through Agrobacterium-mediated transformation or electroporation.
Base editing (such as BE3) and prime editing (PE) enable precise base substitutions while avoiding double-strand breaks. For example, PE is significantly more efficient at achieving single-base mutations in rice than traditional CRISPR.
Multiplex editing technologies (such as Multiplex CRISPR) can simultaneously edit multiple gene loci, for example, achieving synergistic improvements in disease resistance and yield in wheat.
Transformation efficiency can be improved by modifying the Vir region or T-DNA structure of Agrobacterium strains (such as LBA4404 and EHA105). For example, LBA4404 exhibits optimal performance in transforming cotton Fusarium wilt pathogen.
AS is a key inducer for Vir region activation, and its concentration must be precisely controlled to avoid excessive inhibition of plant growth.
Vacuum infiltration improves contact efficiency between Agrobacterium and recipient tissue, while ultrasound-assisted transfection enhances cell membrane permeability. For example, vacuum infiltration increased transformation efficiency to 80% in rice leaf disc transformation.
FACS technology uses flow cytometry to sort cells expressing reporter genes and is suitable for rapid screening after protoplast transformation. For example, in tobacco protoplasts, the sorting efficiency of GFP-positive cells can reach 95%.
The machine vision-based regeneration bud identification system can automate the screening of transformed plants. For example, in a rice regeneration system, this technology has increased screening efficiency by threefold.
Liquid culture combined with microplate technology enables large-scale, high-throughput screening of transformants. For example, liquid culture of rice callus can synchronize cell states and improve screening consistency.
As a model plant, Arabidopsis thaliana's transformation efficiency directly impacts subsequent gene function research. Traditional Agrobacterium-mediated transformation methods (such as the floral dip method) significantly improve transformation success rates by optimizing the bacterial concentration, sucrose, and surfactant ratios (such as Silwet L-77). Furthermore, repeated inoculation and moisturizing treatments can further increase yield, making this method suitable for large-scale T-DNA tagging, gene mapping, and targeted replacement studies.
Transformation of monocots is much more challenging than that of dicots due to their thick cell walls and weak regeneration capacity. Gene gun techniques have achieved efficient transformation of maize by optimizing high-voltage pulse parameters (such as voltage and pulse width) and gold powder particle size distribution. DNA concentration, PEG-mediated cell fusion efficiency, and the MS composition of the screening culture medium are key regulatory factors. By constructing a parameter matrix, quantitative prediction of transformation efficiency can be achieved, providing a standardized approach for genetic improvement of monocot crops.
In the transformation of Populus tomentosa, the ATMT leaf disc method, combined with a synchronized somatic embryogenesis strategy, overcomes the issues of callus browning and low regeneration rates encountered in traditional methods. By manipulating the leaf disc pre-culture time and hormone combination, somatic embryogenesis and adventitious bud differentiation can be simultaneously induced, improving transformation efficiency. This method provides a highly effective tool for forestry genetic improvement, particularly for disease-resistant breeding and wood quality improvement.
In soybean transformation, the "half-seed" method introduces foreign genes directly into ovules, avoiding the lengthy steps of traditional tissue culture. This method increases the yield of T0 generation plants and significantly improves genetic stability compared to conventional methods. By optimizing PEG-mediated protoplast transformation conditions (such as PEG concentration and CaCl2 treatment time), screening cycles can be further shortened, providing an efficient path for the development of herbicide-resistant and high-protein soybean varieties.
Because Catharanthus roseus contains the anticancer drug vincristine, transformation research focuses on the development of transient expression systems. Transient expression of exogenous genes via Agrobacterium infection of leaf discs allows for rapid synthesis of target compounds. Regulating promoter strength and selection marker concentration enables efficient transient expression, providing a new strategy for the in vitro synthesis of terpenoid indole alkaloids.
The genetic stability of transformants must be verified across multiple dimensions. qPCR is used to detect the copy number of the exogenous gene to ensure that there is no accumulation of multiple copies at the insertion site; Southern blot analysis of T-DNA integration sites verifies single-copy integration; and ONT long-read sequencing can comprehensively analyze the risk of genomic rearrangements.
Genetic stability in the T1-T3 generations is assessed through PCR and phenotypic screening (e.g., for resistance and agronomic traits). For example, maize transformants must be screened for three consecutive generations to ensure stable inheritance of the exogenous gene and the absence of phenotypic drift. The genetic stability of monocot transformants is generally lower than that of dicot transformants, necessitating the integration of marker-assisted selection (MAS) to improve efficiency.
The biosafety assessment of transformants must include off-target risks (e.g., CRISPR-Cas9-mediated gene editing) and environmental release testing. For example, soybean transformants must be tested for off-target mutations using target-specific PCR and undergo multi-generational adaptability testing in a closed greenhouse. International regulations (such as the Cartagena Protocol) require environmental risk assessments for genetically modified crops to ensure they have no negative impacts on ecosystems.
Regulation of genetically modified crops varies significantly between countries. The Cartagena Protocol requires risk assessment reports for cross-border shipments of genetically modified materials; the United States Department of Agriculture (USDA) streamlines environmental release approvals for non-food crops through the APHIS waiver process.
DNA-free CRISPR technology delivers RNA polymerase II (RNP) complexes, avoiding the risk of exogenous DNA integration. Nanocarriers (such as liposomes and gold nanoparticles) can improve RNP delivery efficiency and have achieved efficient gene editing in rice and wheat.
Heterologous expression platforms are being constructed by engineering plant photosynthesis systems. For example, industrial enzymes (such as cellulases) are being introduced into tobacco chloroplasts using cyanobacterial photosystem II modules, enabling efficient light-driven production. Such technologies can reduce the cost of traditional fermentation and advance the synthesis of biofuels and chemicals.
Machine learning models (such as random forests and deep learning) can analyze the relationship between transformation parameters (such as electroporation voltage and gene gun speed) and transformation efficiency, predicting optimal conditions.
Plant transformation technology is undergoing a paradigm shift from "art" to "engineering". Standardized processes, AI-assisted design, and breakthroughs in synthetic biology are significantly improving its efficiency and controllability. In the future, interdisciplinary collaboration (such as AI + bioinformatics) and a global service network will further promote the accessibility of this technology and help address global challenges such as food security and ecological restoration.
Lifeasible provides a one-stop transformation platform, encompassing comprehensive technical services from vector construction and genetic transformation to data analysis. We sincerely invite clients worldwide to collaborate with us to advance crop improvement projects. We offer customized transformation solutions and 24/7 online consulting services. Feel free to contact us to discuss project collaboration details.
Q: What is plant transformation technology?
A: Plant transformation technology is a biotechnology method that introduces exogenous DNA into the plant genome for gene expression or genetic improvement. This technology is used to improve crop stress resistance, yield, or added value, promoting agricultural modernization and the development of the biopharmaceutical industry.
Q: What are the core scientific principles of plant transformation?
A: The totipotency and regeneration system of plant cells are the core foundations of plant transformation technology. Under specific conditions, plant cells exhibit totipotency and can differentiate into complete plants, which enables the integration of exogenous genes and the rapid expansion of transgenic plants.
Q: What are the commonly used plant transformation methods?
A: Common methods include Agrobacterium-mediated transformation, gene gun transformation, protoplast PEG-Ca2+ fusion, electroporation, and the pollen tube pathway. Different methods are suitable for different plant types and experimental requirements.
Q: What biological bottlenecks are encountered during plant transformation?
A: Transformation efficiency is limited by factors such as cell wall barriers, host restriction factors, and RNA silencing mechanisms. The cell wall structure and surface receptors that influence transformation vary significantly among plant species.
Q: How can the safety of plant transformation be ensured?
A: Transformants undergo rigorous molecular characterization and phenotypic consistency assessment for genetic stability and biosafety to ensure the absence of off-target effects and environmental hazards.
References