Lifeasible is dedicated to developing plant protection solutions using gene editing technologies, and we provide gene knockout or knockdown services to help achieve plant protection.
Gene knockout is a method of making a gene non-functional at the DNA level, while knockdown is a method to reduce the expression of a gene at the mRNA level. Gene editing enables gene knockout and knockdown to suppress target genes. This can be applied to plant protection. For example, the disease resistance of plants can be increased by suppressing susceptibility genes in plants. The translation initiation factors eIF4E, elF(iso)4E, and eIF4G in plants are susceptibility proteins for certain plant RNA viruses. Gene knockout of a single isoform of these initiation factors can induce resistance to the virus without impacting plant health. Currently, the commonly used knockout method is based on CRISPR/Cas technology (Fig. 1). In addition, gene editing-based host-induced gene silencing (HIGS) is the main application of knockdown in plant protection.
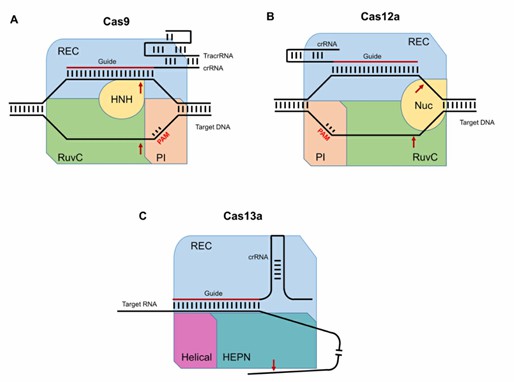 Fig. 1 Schematic representation of the CRISPR/Cas complexes (Kalinina et al., 2020).
Fig. 1 Schematic representation of the CRISPR/Cas complexes (Kalinina et al., 2020).
Knockout or knockdown susceptibility genes in plants
We offer services to knock out or knock down susceptibility genes in plants to improve plant resistance to pathogenic bacteria, fungi, and viruses. If the susceptibility gene does not affect plant survival, we provide knockout services. If the susceptibility gene is related to plant life activities or is indispensable to the plant, we provide gene editing to knock down the susceptibility gene. We can offer multiple gene knockouts or knockdowns, too.
Knockout pest genes
We offer knockout services to alter the survival and reproductive capacity of pests. Knocking out resistance genes of pests can reduce the adaptive capacity of pests, thus reducing the population density of pests. For example, knockout of the P-glycoprotein gene in Spodoptera exigua increases their susceptibility to the pesticides abamectin and emamectin benzoate. In addition, long-term pest control can be achieved by knocking out genes to control the reproductive capacity of pests.
Gene editing-based HIGS
We offer HIGS-based gene knockdown services that leverage the ability of plants to produce small RNAs to silence pest genes, pathogenic fungal genes, and viral genes. This technology combines gene editing and RNA interference technology and is considered to have promising applications.
Gene knockout or knockdown for exploring plant protection
We offer knockout or knockdown services for genes in plants, pests, and pathogenic fungi of unknown function to help explore gene knockouts or knockdowns that may contribute to plant protection.
For knockout in plants, we offer methods using CRISPR/Cas technology, TALEN technology, and ZFNs technology. We have extensive experience in using a wide range of virus-based CRISPR/Cas9 genome editing. For knockdown in plants, we can achieve knockdown based on gene editing and non-gene editing approaches. Here, we offer various gene editing methods to insert interference genes into the plant genome to express miRNAs or shRNAs to silence target gene expression. Our knockout and knockdown services are optimized and validated to ensure the success and efficiency of the experiments.
For knockout in pests, we mainly use CRISPR/Cas9 technology. And we use the embryo injection method for transformation.
We mainly use CRISPR/Cas9 and CRISPR/Cas12a technology to knock out genes of pathogenic fungi, including filamentous fungi and pathogenic oomycetes.
Lifeasible is dedicated to helping develop plant protection strategies using knockout or knockdown technologies. Please do not hesitate to contact us for your plant protection-related gene knockout or knockdown program.
References
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
STU-CRISPR System Improves Plant Genome Editing Efficiency
April 19, 2024
Application of Exosomes in Facial Beauty
April 12, 2024