The ubiquitin system is involved in numerous important cellular processes in eukaryotes. Lifeasible helps to investigate the role of the ubiquitin system and protein ubiquitination in plant-pathogen interactions.
The ubiquitin system plays a key role in the regulation of plant-pathogen interactions. The ubiquitin system is closely associated with plant resistance gene-mediated resistance, pathogen microbe-associated molecular pattern (PAMP)-triggered immunity (PTI) (Fig. 1), effector-triggered immunity (ETI), and programmed cell death (PCD). In turn, pathogens hijack the plant ubiquitin system to increase its virulence. For eukaryotic pathogens, the ubiquitin system functions in their growth and pathogenicity. The ubiquitin system mediates protein ubiquitination. Ubiquitination is an essential protein post-translational modification (PTM) in eukaryotes that regulates multiple cellular processes through 26S proteasome-mediated protein degradation or affects protein-protein interactions and localization. In summary, there is a strong need to investigate the role of the ubiquitin system and protein ubiquitination in plant-pathogen interactions.
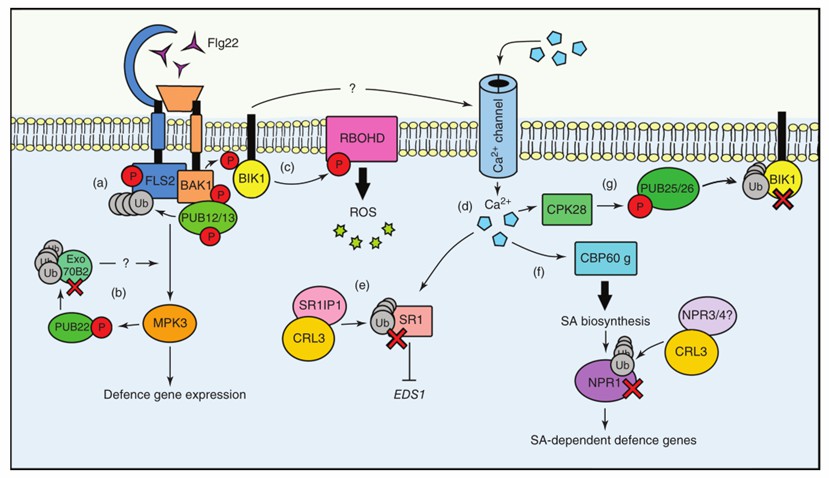 Fig. 1 The roles of the ubiquitin system during PTI signaling (Sorel et al., 2019).
Fig. 1 The roles of the ubiquitin system during PTI signaling (Sorel et al., 2019).
Lifeasible helps to investigate the role of the ubiquitin system and protein ubiquitination in plant-pathogen interactions. We help explore the effects of proteins or molecules in plants/pathogens on the plant/pathogen ubiquitin system, and we help detect the effects of protein ubiquitination on plant-pathogen interactions.
Detection of protein ubiquitination
We help detect protein ubiquitination that occurs in plant-pathogen interactions. This will facilitate the determination of the functional status of the ubiquitin system and the identification of substrates for the ubiquitin system.
We help detect protein ubiquitination based on specific antibodies by western blot and immunoprecipitations. We help extract, purify and isolate proteins, which are then bound with specific antibodies and photographed to analyze protein ubiquitination.
We help detect protein ubiquitination based on mass spectrometry (MS). We help extract and enzymatically digest protein samples and affinity enrich for ubiquitinated peptides. We then help with the analysis using liquid chromatography (LC)-MS/MS. We will perform comparative analysis and bioinformatics analysis of the mass spectrometry data to qualitatively and quantitatively identify protein ubiquitination.
Validation of protein ubiquitination
E3 ubiquitin ligase is essential for specific and selective ubiquitination, and E2 ubiquitin-coupled enzyme or the E2-E3 complex also determines the synthesis of polyubiquitin chains. We help identify the specific E3, E2, and ubiquitin substrates in protein ubiquitination. We help express and collect the proteins (E3, E2, or ubiquitin substrates) to be tested and incubate them in vitro to validate protein ubiquitination. We then subject the incubated products to immunoprecipitation and western blot analysis to determine the E3 and E2 that cause ubiquitination modifications to a particular protein.
Detecting interactions with the ubiquitin system
Some effectors of pathogens increase their virulence by affecting the function of the ubiquitin system, and we help to detect how these effectors interact with the ubiquitin system. We master various protein-protein interaction research techniques to help achieve the study of such interactions.
Functional studies of ubiquitinated proteins
We help to investigate the role of degradable ubiquitinylated proteins mainly by gene knockout and RNA interference. For the function of non-degradable ubiquitinated proteins, we will help explore their specific functions through gene editing, protein localization, and protein interaction studies.
Lifeasible offers professional services to help study the role of the ubiquitin system and protein ubiquitination in plant-pathogen interactions. Please contact us to advance your project.
References
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
STU-CRISPR System Improves Plant Genome Editing Efficiency
April 19, 2024
Application of Exosomes in Facial Beauty
April 12, 2024