Seed storage proteins are synthesized as precursors on the endoplasmic reticulum (ER) and then transported to protein storage vacuoles, where they are processed into mature forms. MAG2 body is a novel structure found in cells, containing storage protein precursors and endoplasmic reticulum molecular chaperones. It has a core part with high electron density and a matrix part with low electron density. The core fraction consists of precursors of 2S albumin, and the matrix fraction contains precursors of 12S globulin as well as immunoglobulin heavy chain-binding protein and protein disulfide isomerase.
Lifeasible provides functional analysis of the MAG2 body, including storage protein precursors and molecular chaperones, to help our customers worldwide in the field of plant science. Our platform is equipped with cutting-edge facilities and professional experts to support research. Here, we provide various services according to customers' demands.
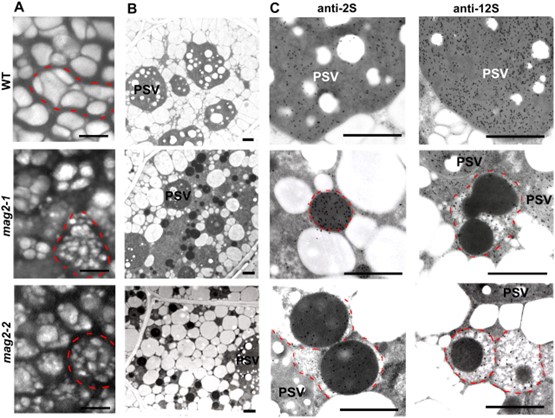 Fig.1. MAG2 Arabidopsis mutant seeds develop a number of novel structures that accumulate storage proteins. (Li L., et al., 2006)
Fig.1. MAG2 Arabidopsis mutant seeds develop a number of novel structures that accumulate storage proteins. (Li L., et al., 2006)
Lifeasible provides cost-effect, high quality, and hassle-free services to our clients worldwide. We provide our clients with direct access to our experts and prompt response to their inquiries. If you are interested in our services or have any questions, please feel free to contact us or make an online inquiry.
Reference
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
STU-CRISPR System Improves Plant Genome Editing Efficiency
April 19, 2024
Application of Exosomes in Facial Beauty
April 12, 2024