Lifeasible facilitates the study of the interaction between effector proteins and other proteins and provides various services to help study the interaction mechanism between effector proteins and other proteins.
Effectors are an important component of plant-pathogen interactions, and most effectors are proteins containing 50-300 amino acid residues. They can interfere with the physiological activities of the plant (Fig. 1). They exert their pathogenic effects mainly by targeting resistance proteins in the plant. Plant-pathogen interactions can be described as a high-speed evolutionary arms race, with many effectors involved in all stages of pathogen infestation. The current research on effectors and effector mechanisms is only the top of the "effector iceberg". Lifeasible contributes to the study of effector interactions and provides services to support the study of interactions and interaction mechanisms of effector proteins, which account for a high proportion of effectors.
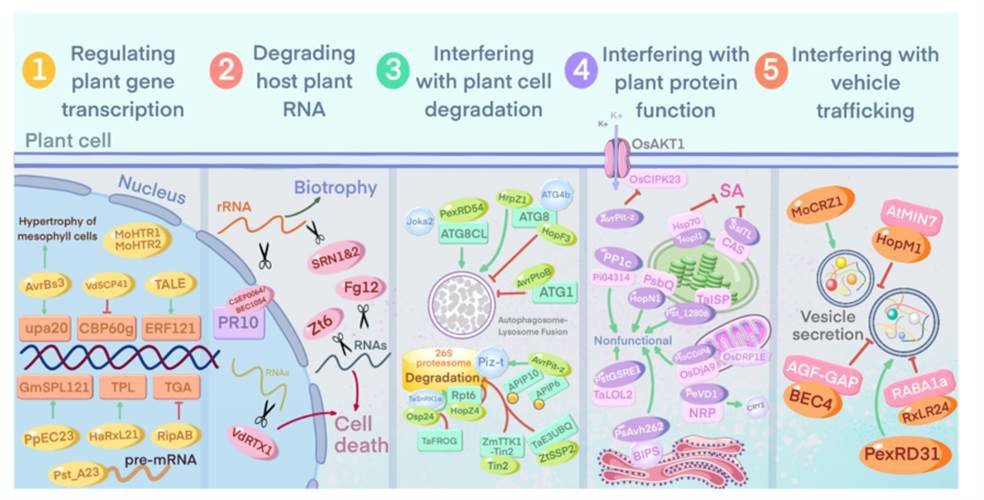 Fig. 1 Effectors interfere with host plant cell physiological activities (Zhang et al., 2022).
Fig. 1 Effectors interfere with host plant cell physiological activities (Zhang et al., 2022).
Lifeasible mainly helps to study the interaction between effector proteins and plant proteins and helps to explore the mechanism of action between them. For interactions between effector proteins that work in concert, we can also provide interaction studies. We master various advanced methods to help study protein-protein interactions.
Affinity purification-mass spectrometry (AP-MS)
For two proteins with strong affinity, we can offer interaction validation using AP-MS methods. This method can be used to validate effector proteins that act as plant protease inhibitors. Plant protease inhibitors have strong interaction with plant proteases. The method helps to discover a larger number of effector protein inhibition targets.
Co-immunoprecipitation technology
Immunoprecipitation is used to screen for effector interactors in heterologous systems. We can help detect interactions between effector proteins and plant proteins using co-immunoprecipitation technology. After immunoprecipitation, we offer analysis using mass spectrometry (MS) or fluid chromatography-mass spectrometry (LC-MS) coupling.
Split-marker complementation technology
We can offer bifurcated fluorescence complementation (BiFC) and luciferase complementation imaging (LCI) methods to help validate the interaction between effector proteins and plant proteins. Of the two methods, we recommend the LCI method. This method is unaffected by autofluorescence produced by the plant cell wall and chlorophyll and allows whole tissues and cell populations to be sampled. In terms of data collecting, its data can be collected in 1-2 min using only a plant living imaging system and plate luminescence detector. What's more, the data obtained by this method can be used for quantitative analysis.
Proximity labeling technology
For proteins with weak or transient interactions, we help to validate the interactions using proximity labeling technology. Biotinylation is a rare protein modification in nature. BioID and TurboID can biotinylate other proteins and allow for labeling spatially adjacent proteins. We offer the use of them for effector protein-plant protein interaction studies. We prefer to use TurboID, which has been shown to provide more efficient labeling in planta and can also reduce the biotin incubation time from 16 h to 10 min.
Other methods
We also master other methods for effector protein-plant protein interaction studies, including fluorescence resonance energy transfer and yeast two-hybrid techniques. We have cryo-electron microscopy to help analyze the structure of protein complexes. In addition, we have mastered Agrobacterium-mediated transient transfection to help study effector protein-plant protein interactions.
Lifeasible is capable of implementing effector protein-plant protein interaction studies using a combination of various protein interaction study techniques. Please contact us for the most professional service.
References
Lifeasible has established a one-stop service platform for plants. In addition to obtaining customized solutions for plant genetic engineering, customers can also conduct follow-up analysis and research on plants through our analysis platform. The analytical services we provide include but are not limited to the following:
STU-CRISPR System Improves Plant Genome Editing Efficiency
April 19, 2024
Application of Exosomes in Facial Beauty
April 12, 2024